Ciencia y Salud, Vol. 9, No. 3, septiembre-diciembre, 2025 • ISSN (impreso): 2613-8816 • ISSN (en línea): 2613-8824
CLINICAL AND EPIDEMIOLOGICAL PROFILE OF PEDIATRIC MYCOPLASMA PNEUMONIAE INFECTIONS IN SANTO DOMINGO
Perfil Clínico y Epidemiológico de Infecciones Pediátricas por Mycoplasma pneumoniae en Santo Domingo
DOI: https://doi.org/10.22206/cisa.2025.v9i3.3436
Rafael Mena1, Alam L.2, Mirtha Calderón3, María Laura Garcia4, Camila Guzmán Basilis5, María Alejandra Ubiera6, Esperanza Mendoza7
Recibido: 15/1/2025 ● Aceptado: 04/2/2025
Cómo citar: Mena, R., Alam, L., Calderón, M. García, M. L., Guzmán Basilis, C., Ubiera, M. A., & Mendoza, E. (2025). Clinical and Epidemiological Profile of Pediatric Mycoplasma pneumoniae Infections in Santo Domingo. Ciencia y Salud, 9(3), 5-15. https://doi.org/10.22206/cisa.2025.v9i3.3436
Abstract
Introduction: There is limited research on Mycoplasma pneumoniae in resource-limited settings. Observing the high variability of signs and symptoms of this infection in the pediatric population and its similarity to autoimmune pathologies, a retrospective, observational, descriptive and cross-sectional study was conducted. Methods: This study analyzed the epidemiology, clinical characteristics, diagnosis and treatment of M. pneumoniae infection in 100 pediatric patients who presented with the condition and were treated in a private pediatric clinic in the Dominican Republic from January 2023 to July 2024. The primary purpose of this study was to enhance physicians’ ability to accurately identify and diagnose the condition, thereby preventing potential complications. Results: The study cohort had a mean age of 7 years and 9 months and was 52% male, 84% of the patients were diagnosed with Mycoplasma pneumoniae infection based on elevated IgM antibody levels. Fever was the most common systemic symptom, occurring in 51% of cases, followed by cough and rash, each present in 49% of patients. Pulmonary findings were observed in 23% of the patients. Notably, 59% exhibited extrapulmonary manifestations, with pharyngitis being the most prevalent among them. All patients were treated with a standardized azithromycin regimen. Conclusions: This study highlights the urgent need for comprehensive diagnostic and treatment protocols, especially in resource-limited settings, to address the complexity of coinfections and develop equitable, evidence-based global guidelines.
Keywords: Fever, Mycoplasma pneumoniae, pediatrics, pharyngitis, rash.
Resumen
Introducción: Existe poca investigación sobre Mycoplasma pneumoniae en entornos de recursos limitados. Ante la alta variabilidad de los signos y síntomas de esta infección en la población pediátrica y su similitud con las patologías autoinmunes, se realizó un estudio retrospectivo, observacional, descriptivo y transversal. Métodos: Este estudio analizó la epidemiología, las características clínicas, el diagnóstico y el tratamiento de la infección por M. pneumoniae en 100 pacientes pediátricos que presentaron la afección y fueron tratados en una clínica pediátrica privada en la República Dominicana entre enero de 2023 y julio de 2024. El objetivo principal de este estudio fue mejorar la capacidad de los médicos para identificar y diagnosticar con precisión la afección, previniendo así posibles complicaciones. Resultados: La cohorte del estudio tenía una edad media de 7 años y 9 meses y el 52% eran varones. El 84% de los pacientes fueron diagnosticados con infección por Mycoplasma pneumoniae basándose en niveles elevados de anticuerpos IgM. La fiebre fue el síntoma sistémico más frecuente, presente en el 51% de los casos, seguida de la tos y el exantema, ambos presentes en el 49% de los pacientes. Se observaron hallazgos pulmonares en el 23% de los pacientes. Cabe destacar que el 59% presentó manifestaciones extrapulmonares, siendo la faringitis la más prevalente. Todos los pacientes recibieron tratamiento con un régimen estandarizado de azitromicina. Conclusiones: Este estudio destaca la urgente necesidad de protocolos integrales de diagnóstico y tratamiento, especialmente en entornos con recursos limitados, para abordar la complejidad de las coinfecciones y desarrollar directrices globales equitativas y basadas en la evidencia.
Palabras clave: Fiebre, Mycoplasma pneumoniae, pediatría, faringitis, exantema.
Introduction
Globally, M. pneumoniae remains a significant pathogen in pediatric respiratory infections, yet its diagnostic and management challenges are exacerbated in resource-constrained settings where laboratory facilities are limited. Mycoplasma pneumoniae is a significant contributor to respiratory tract infections, particularly in children1–3. It can present with a wide range of clinical symptoms, from being asymptomatic to causing severe pneumonia or extrapulmonary diseases1, 2. It is also one of the main pathogens responsible for community-acquired pneumonia1, 13. Most cases are observed in patients over five years of age, while infections in younger children tend to be less severe2. Around 25% of children with an M. pneumoniae infection experience extrapulmonary complications, including conditions such as myocarditis, hepatitis, encephalitis, thrombocytopenic purpura, autoimmune hemolysis, and damage to the skin and mucous membranes. The low frequency of detection of M. pneumoniae in non-pulmonary samples suggests that these extrapulmonary effects are likely driven by the immune response to the infection2. This pathogen is transmitted through close personal contact via airborne particles and respiratory droplets, particularly within familial, educational, military, institutional, and other close-knit community settings3.
Mucocutaneous eruptions caused by M. pneumoniae are observed in around 25% of pediatric patients. Mycoplasma-induced rash and mucositis (MIRM) is a rare condition characterized by mucositis and distinctive, sparse, vesiculobullous, or target-like skin eruptions3, 4. The underlying mechanisms of MIRM differ from those of erythema multiforme, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN)14. Despite some similarities in the rashes, treatment approaches vary3. Due to its rarity, diagnosing MIRM can be a challenge for physicians, who may struggle to differentiate it from similar conditions5, 6.
M. pneumoniae infections have been observed to occur both endemically across diverse global climates and epidemically at intervals of several years. Previous epidemics in Europe were documented in the periods of 2010-2012, 2014-2015, and 2015-2017. Recent reports from the Centers for Disease Control and Prevention in the United States indicate that the prevalence of Mycoplasma pneumoniae infections among children has been on the rise. The data shows an increase from 1% in March to a sustained high of 7.2% by October 2024. This calls for continued vigilance and improved diagnostic capabilities, particularly in low-resource settings where the burden of pediatric respiratory infections is highest3.
Despite the fact that M. pneumoniae-associated erythema multiforme has been documented globally, there is limited evidence of its prevalence or clinical management in the Dominican Republic14. Additionally, a gap exists in understanding potential coinfections and complications associated with M. pneumoniae, which may contribute to diagnostic challenges and suboptimal patient outcomes. This lack of data highlights the need for region-specific research to address these gaps and improve clinical awareness.
This study seeks to address this problem by retrospectively analyzing the epidemiology, clinical characteristics, diagnostic challenges, and management of M. pneumoniae infections in 100 pediatric patients treated at a private clinic in Santo Domingo, Dominican Republic, between January 2023 and July 2024. By identifying patterns and gaps in diagnosis and care, this research aims to enhance healthcare professionals' capacity to accurately diagnose and manage M. pneumoniae-associated conditions, ultimately reducing the risk of complications and improving outcomes in resource-limited settings.
Materials and methods
This observational, descriptive, cross-sectional study was conducted in the Pediatrics Departments of two private clinics, La Cliniquita and Centro de Obstetricia y Ginecología, in Santo Domingo, Dominican Republic. The study included pediatric patients (aged 0 to 15 years) presenting with fever, cough, respiratory symptoms, and mucocutaneous lesions between January 2023 and July 2024. Inclusion criteria required patients to exhibit clinical signs consistent with Mycoplasma pneumoniae infection. Patients with incomplete medical records or those lacking laboratory or imaging results were excluded.
A convenience sampling method was used, with data retrospectively collected from medical records available at the participating clinics. Patient demographics (age, sex), clinical features, hospitalization details, treatment response, coinfections, and laboratory and imaging results (chest X-rays, CT scans) were extracted. Access to patient records followed institutional guidelines, ensuring confidentiality and compliance with ethical standards. All diagnostic and clinical procedures adhered to established pediatric care protocols.
Results
Clinical presentation
A total of 100 patients were included in the study, with an average age of 7 years and 9 months, the youngest being 7 months and the oldest 15 years. Males predominated, comprising 52% of the cases, compared to 48% of females. Among the systemic symptoms, fever was the most common, occurring in 51% of the sample, followed by both cough and rash at 49% each. Respiratory findings were present in 23% of the patients. A deeper analysis revealed that 59% of the population sample exhibited various extrapulmonary symptoms, with pharyngitis being the most common, found in 11 (18.63%) of these patients, followed by vomiting and nasal congestion, both observed in 16.95% (10) of the patients with extrapulmonary symptoms.
Cutaneous findings
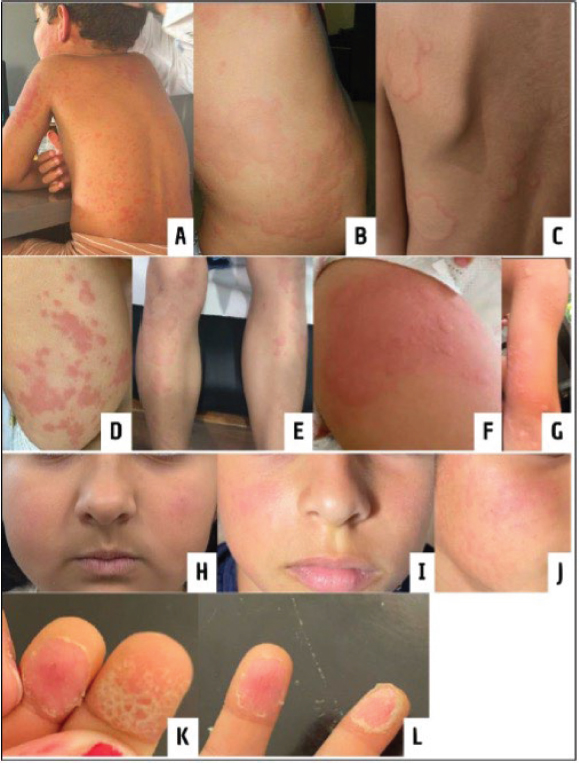
Among the 100 patients, nearly half (49%) exhibited cutaneous manifestations. These lesions displayed a diverse range of morphologies, including erythematous and widespread presentations with a sudden, disorganized appearance in some cases. In other instances, isolated flat erythematous facial lesions were observed, akin to the malar rash characteristic of certain autoimmune conditions. Additionally, some patients presented with peeling of the fingers. The most commonly affected anatomical areas by these cutaneous lesions were the torso, extremities, and face (Figure 1).
Figure 1. Polymorphic, erythematous, and widespread lesions on the torso (A-C) and extremities (D-G). Flat erythematous facial lesions (H-J). Peeling of the distal phalanx of the second and third fingers on the left hand (K-L)
Pulmonary findings and Chest X-Rays
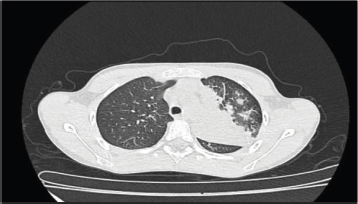
Respiratory findings were observed in 23% of patients, including crackles, wheezing, rhonchi, crepitations, reduced air entry and exit during inspiration and expiration, and decreased vesicular breath sounds on physical examination. Of these, isolated rhonchi, detected in 6 (26.1%) of the 23 patients, were the most common pulmonary finding. Chest X-rays were performed on 7% of the study population, revealing pneumonia in three (3) cases—one in the right lung and two in the left lung (Figure 2). One patient with left lung pneumonia also required a chest CT scan (Figure 3).
Figure 2. Pneumonia in the right lung (A) and in the left lung (B-C)
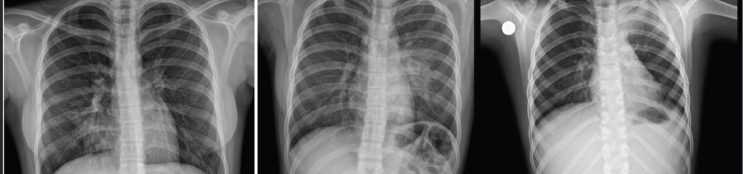
Figure 3. Pulmonary consolidation with multiple foci in the left upper and lower lobes
Laboratory findings
The study utilized two diagnostic methods: the FilmArray Respiratory Panel and measurement of IgM levels for M. pneumoniae. All cases examined returned positive results. Additionally, coinfections with other respiratory pathogens, including rhinovirus, metapneumovirus, influenza virus, cytomegalovirus, and coronavirus, were identified. The majority of cases, 84%, were diagnosed through IgM testing for Mycoplasma, while 22% were identified solely by the FilmArray Respiratory Panel, and 6% were diagnosed using both approaches.
Treatment
All participants in the study received a 5-day course of oral azithromycin, with an initial dose of 10 mg/kg followed by 5 mg/kg for the remaining 4 days. Additionally, the majority of patients received supportive care with antipyretics, and some also required antihistamines and corticosteroids. Notably, only a single patient exhibited complications requiring hospitalization.
Discussion
The demographic analysis revealed a slight male predominance, with males comprising 52% of the cases compared to 48% females. This gender distribution is consistent with prior studies, which have reported a relatively even spread among pediatric patients with M. pneumoniae infections6. The mean age of 7 years and 9 months suggests this infection affects a broad age spectrum, from infants to adolescents, underscoring the necessity for continuous clinical monitoring across the entire pediatric population7, 8.
The findings indicate that fever was the most common systemic symptom, observed in 51% of the patient cohort. This is consistent with previous research, wherein fever frequently serves as a primary marker of Mycoplasma pneumoniae infection8. Additionally, cough and rash were present in 49% of the sample, further underscoring the respiratory and cutaneous manifestations characteristic of this condition7, 8. The high incidence of rash emphasizes the importance of considering M. pneumoniae in the differential diagnosis of pediatric patients presenting with fever and skin abnormalities, especially when other common causes are absent2.
Pathological respiratory findings were present in 23% of the participants, with wheezing, rhonchi, and crackles being the most common manifestations. Among these respiratory abnormalities, isolated rhonchi were the most frequently observed, occurring in 26.1% of patients. These results emphasize the substantial respiratory involvement associated with M. pneumoniae infections, which can resemble other respiratory conditions, potentially hindering accurate diagnosis9. Furthermore, the reduced air entry and diminished vesicular breath sounds identified during physical examinations underline the importance of a comprehensive respiratory assessment in suspected cases7, 8.
Extrapulmonary manifestations were common, affecting 59% of patients, beyond just respiratory symptoms. The most prevalent extrapulmonary symptoms were pharyngitis (18.63%), vomiting (16.95%), and nasal congestion (16.95%) (Table 1). These findings align with M. pneumoniae’s capacity to impact multiple organ systems, emphasizing the need for a comprehensive clinical assessment when managing this infection in pediatric patients. Additionally, other diverse extrapulmonary symptoms, such as changes in bowel habits, abdominal pain, fatigue, and joint pain, further highlight the variable clinical presentation of M. pneumoniae in the pediatric population.
Table 1. Extrapulmonary manifestations presented by patients caused by M. pneumoniae
Extrapulmonary Manifestations of M. pneumoniae |
||
Findings |
Frequency |
% of Total |
Pharyngitis |
11 |
18.64% |
Vomiting |
10 |
16.95% |
Nasal congestion |
10 |
16.95% |
Fatigue/Weakness |
9 |
15.25% |
Abdominal pain |
8 |
13.56% |
Otitis |
7 |
11.86% |
Tonsillitis |
7 |
11.86% |
Body aches |
6 |
10.17% |
Headache |
6 |
10.17% |
Constipation/Diarrhea |
4 |
6.78% |
Rhinorrhea |
3 |
5.08% |
Anorexia |
3 |
5.08% |
Cervical adenopathy |
3 |
5.08% |
Dizziness |
3 |
5.08% |
Itching (Pruritus) |
2 |
3.39% |
Hives (Urticaria) |
2 |
3.39% |
Pale mucous membranes |
2 |
3.39% |
Tearing (Lacrimation) |
1 |
1.69% |
Dysuria |
1 |
1.69% |
Insomnia |
1 |
1.69% |
Weight loss |
1 |
1.69% |
Hyperemic nasal turbinates |
1 |
1.69% |
Thrombocytopenia |
1 |
1.69% |
Respiratory diffculty |
1 |
1.69% |
Total |
100% |
|
Cutaneous manifestations were exhibited by nearly half of the patients, characterized by a diverse range of lesion morphologies, including polymorphic, erythematous, and widespread presentations. Notably, some cases displayed a sudden and disorganized appearance of these skin lesions, while others presented with isolated erythematous facial lesions akin to the malar rash observed in certain autoimmune conditions2. Additionally, the observation of peeling in some patients further underscores the varied spectrum of cutaneous symptoms associated with M. pneumoniae infections.
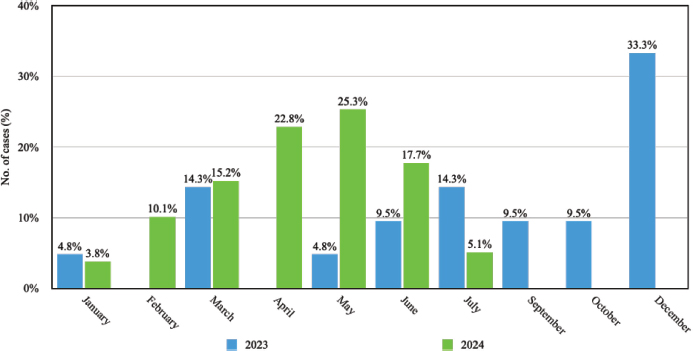
Distinct seasonal patterns in M. pneumoniae’s incidence of infections were observed within the study cohort. In 2023, the highest prevalence was recorded in December, representing 33.3% of cases, while in 2024, the peak occurred in May, accounting for 25.3% of the infections (Figure 4). A study by Cheng et al. in Beijing, China, reported a different seasonal distribution of M. pneumoniae infections, with peaks in August, January, and October10, in contrast to the findings of the present study. This divergence may be attributable to regional climatic differences, variations in population density, or disparities in post-pandemic public health measures. For example, the tropical climate of the Dominican Republic, characterized by relatively constant temperature and high humidity, may influence transmission dynamics differently compared to the temperate climate of Beijing, where seasonal temperature fluctuations are more pronounced. The winter spike could be tied to increased indoor gatherings and travel during the holiday season, which could have aided the pathogen’s spread.
Figure 4. Monthly distribution of M. pneumoniae cases in 2023 and 2024
The variability in seasonal patterns of M. pneumoniae infections highlights the significance of local epidemiological monitoring to guide tailored public health interventions. Conducting comparative investigations across diverse geographical regions and climatic zones is essential to elucidate the underlying determinants driving these disparities, which in turn will enable more accurate forecasting and the development of effective management strategies for this pathogen.
Among all patients, 8 presented coinfections with other pathogens, and one of them had a coinfection with two additional microorganisms besides M. pneumoniae. Rhinovirus was the most common pathogen (4 cases), followed by influenza A (2), metapneumovirus (1), OC43 variant coronavirus (1), and cytomegalovirus (1). In a study by Lai S. et al. (2024), it was found that out of 142 patients, 16 (13%) had coinfections. Similar to our findings, rhinovirus (9) was the most frequent pathogen in these coinfections2. This underscores the potential for M. pneumoniae to coexist with other respiratory infections, which can complicate both the clinical presentation and its management11,12.
The high prevalence of rhinovirus coinfection in M. pneumoniae cases highlights the significant role this pathogen plays as a coinfecting agent. Rhinovirus is known for its high transmissibility and prevalence in pediatric respiratory illnesses, which may contribute to its frequent association with M. pneumoniae. The coexistence of these pathogens can complicate the clinical presentation, as symptoms such as cough, fever, and respiratory distress may overlap, making it challenging for clinicians to attribute specific manifestations to a single pathogen. This highlights the importance of comprehensive diagnostic panels to accurately identify coinfections and guide appropriate treatment strategies.
The presence of influenza A, metapneumovirus, and coronavirus OC43 in our cohort further illustrates the diverse range of pathogens that can co-occur with M. pneumoniae. These findings highlight the potential for overlapping epidemics during certain seasons, necessitating heightened clinical vigilance. Coinfections may also exacerbate disease severity, as reported in other studies where viral coinfections were associated with prolonged hospital stays and increased morbidity.
Due to the lack of a cell wall in M. pneumoniae, antimicrobials targeting the cell wall, such as penicillin, are ineffective. Macrolides, including azithromycin and clarithromycin, are considered the primary antibiotic treatments for pediatric patients. Tetracyclines, like doxycycline, are reserved for severe cases in children, while quinolones, such as levofloxacin and ciprofloxacin, are not recommended for individuals under 18 years old5. The five-day treatment with azithromycin proved effective, with most patients responding positively to the regimen. The utilization of symptomatic therapies, encompassing antipyretics, antihistamines, and steroids in several cases, shows the importance of addressing both the infection and its associated symptoms. Notably, only one patient experienced complications necessitating hospitalization, indicating that early and appropriate intervention can prevent severe outcomes in the majority of cases.
Conclusion
This study highlights the clinical and epidemiological characteristics of Mycoplasma pneumoniae infections in pediatric patients treated in a resource-limited setting. The findings underscore the broad clinical spectrum of the disease, including systemic, respiratory, and extrapulmonary manifestations, with a notable prevalence of cutaneous and mucocutaneous symptoms. The observed seasonal variability suggests the influence of local climatic and social factors on transmission dynamics, emphasizing the importance of region-specific epidemiological monitoring.
The coexistence of coinfections, particularly with rhinovirus, influenza A, and other respiratory pathogens, complicates the clinical presentation and reinforces the need for comprehensive diagnostic strategies to improve accuracy. The effective response to macrolide-based treatment highlights the importance of early diagnosis and appropriate antimicrobial therapy in preventing severe outcomes.
These results contribute to a deeper understanding of the clinical and epidemiological patterns of M. pneumoniae in pediatric populations and provide a foundation for tailored public health interventions and improved clinical management in similar settings. Further research is warranted to explore the long-term impact of coinfections and the role of local environmental factors in disease incidence.
Acknowledgments
The authors would like to thank all the patients and their families for their participation in this study and for sharing their experiences for this publication. They also wish to thank the Centro de Obstetricia y Ginecología and La Cliniquita for their support of this study.
Funding
This research did not receive any specific grant from public, commercial, or nonprofit funding agencies.
Author contributions
Rafael Mena: Responsible for conceptualization and design of the idea, methodology, supervision and follow-up, and provided final approval of the work for publication. Mirtha Calderón: Contributed to the conceptualization of the idea, supervision and follow-up, and also provided final approval of the work for publication. María Laura García: Responsible for methodology, formal analysis, data curation, manuscript drafting, and provided final approval of the work for publication. Camila Guzmán Basilis: Contributed to methodology, formal analysis, data curation, manuscript drafting, and provided final approval of the work for publication. María Alejandra Ubiera: Responsible for methodology, formal analysis, data curation, manuscript drafting, and provided final approval of the work for publication. Esperanza Mendoza: Contributed to conceptualization of the idea, methodology, supervision and follow-up, formal analysis, manuscript drafting, and provided final approval of the work for publication.
Ethical statement
All participating families provided informed consent for the use of photographs of the children included in this paper. Since this is a retrospective study, no additional ethical requirements were necessary.
We declare that this manuscript is original, has not been previously published, and is not currently under consideration for publication elsewhere. We have no knowledge of conflicts of interest associated with this publication, and there was no financial support for this work that could have influenced its outcomes.
We confirm that the manuscript has been read and approved for submission by all the listed authors.
Disclaimer
The conclusions of this article are the sole responsibility of the authors and do not necessarily reflect the opinions, policies, or positions of Ciencia y Salud, its editors, or the Instituto Tecnológico de Santo Domingo (INTEC).
References
1. Youn Y-S, Lee K-Y. Mycoplasma pneumoniae pneumonia in children. Korean J Pediatr. 2012;55(2):42. https://doi.org/10.3345/kjp.2012.55.2.42
2. Chen N, Li M. Case Report and Literature Review: Clinical Characteristics of 10 Children With Mycoplasma pneumoniae-Induced Rash and Mucositis. Front Pediatr. 2022;10(March):1-9. https://doi.org/10.3389/fped.2022.823376
3. Meyer Sauteur PM, Beeton ML, Uldum SA, et al. Mycoplasma pneumoniae detections before and during the COVID-19 pandemic: results of a global survey, 2017 to 2021. Eurosurveillance. 2022;27(19). https://doi.org/10.2807/1560-7917.ES.2022.27.19.2100746
4. Lofgren DH, Lenkeit C, Palanisamy J, Brown J. Mycoplasma Pneumoniae Induced Rash and Mucositis with Bilateral Otitis Media and Sinusitis. Cureus. 2020;12(3). doi:10.7759/cureus.7449
5. Søndergaard MJ, Friis MB, Hansen DS, Jørgensen IM. Clinical manifestations in infants and children with Mycoplasma pneumoniae infection. Schildgen O, ed. PLoS One. 2018;13(4):e0195288. https://doi.org/10.1371/journal.pone.0195288
6. Gao L, Sun Y. Laboratory diagnosis and treatment of Mycoplasma pneumoniae infection in children: a review. Ann Med. 2024;56(1). https://doi.org/10.1080/07853890.2024.2386636
7. Li J, Zhang H, Guo J, Ma X. Clinical features of Mycoplasma pneumoniae pneumonia in children without fever. BMC Pediatr. 2024;24(1):52. https://doi.org/10.1186/s12887-023-04512-1
8. Zhao M, Wang L, Qiu F, et al. Impact and clinical profiles of Mycoplasma pneumoniae co-detection in childhood community-acquired pneumonia. BMC Infect Dis. 2019;19(1):835. https://doi.org/10.1186/s12879-019-4426-0
9. Zhang X-B, He W, Gui Y-H, et al. Current Mycoplasma pneumoniae epidemic among children in Shanghai: unusual pneumonia caused by usual pathogen. World J Pediatr. 2024;20(1):5-10. https://doi.org/10.1007/s12519-023-00793-9
10. Cheng Y, Cheng Y, Dai S, et al. The Prevalence of Mycoplasma Pneumoniae Among Children in Beijing Before and During the COVID-19 Pandemic. Front Cell Infect Microbiol. 2022;12(April):1-6. https://doi.org/10.3389/fcimb.2022.854505
11. Lai SHF, Kuok MCI, Ho PPK, Yau YS. Mycoplasma pneumoniae and Viral Pneumonia Coinfection: Something NOT to be Overlooked. Pediatr Infect Dis J. 2024;43(5):e191-e191. https://doi.org/10.1097/INF.0000000000004249
12. Lee KY. Pediatric respiratory infections by Mycoplasma pneumoniae. Expert Rev Anti Infect Ther. 2008 Aug;6(4):509-21. https://doi.org/10.1586/14787210.6.4.509. PMID: 18662117
13. Valle J, Nasrollahi F, Eilbert W. Mycoplasma pneumoniae-induced rash and mucositis. Am J Emerg Med. 2022 Apr;54:324.e5-324.e7. https://doi.org/10.1016/j.ajem.2021.09.080. Epub 2021 Oct 6. PMID: 34642080.
14. Mena R, Calderón M, Mendoza E, Aira K. Eritema Multiforme Inducido por Mycoplasma pneumoniae: características clínicas en ocho pacientes. ADOPA. 2023;1(2). https://doi.org/10.58994/adopa.v1i2.18
_______________________________
1 MD. FAAP. MBA. Unidad de Cuidados Intensivos Neonatal, Centro de Obstetricia y Ginecología, Santo Domingo, República Dominicana. Division of Neonatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA. ORCID: https://orcid.org/0000-0003-4003-999X, email: rafael.mena@cchmc.org
2 MD, Pediatra Neumólogo, Clínica Corazones Unidos, Santo Domingo, República Dominicana y Universidad Iberoamericana (UNIBE), Santo Domingo, República Dominicana. ORCID: https://orcid.org/0009-0003-2500-2205, email: dr.alam@claro.net.do
3 MD. Unidad de Cuidados Intensivos Neonatal, Centro de Obstetricia y Ginecología, Santo Domingo, República Dominicana. ORCID: https://orcid.org/0000-0002-9173-4506, email: talmi_ch@msn.com
4 MD. Unidad de Cuidados Intensivos Neonatal, Centro de Obstetricia y Ginecología, Santo Domingo, República Dominicana. ORCID: https://orcid.org/0009-0000-6077-6159, email: marilaugarciacordero@gmail.com
5 MD. Unidad de Cuidados Intensivos Neonatal, Centro de Obstetricia y Ginecología, Santo Domingo, República Dominicana. ORCID: https://orcid.org/0009-0008-7780-9912, email: camilaguzmanbasilis@gmail.com
6 MD. Unidad de Cuidados Intensivos Neonatal, Centro de Obstetricia y Ginecología, Santo Domingo, República Dominicana. ORCID: https://orcid.org/0009-0004-4458-9336, email: maria.maug20@gmail.com
7 MS. Unidad de Cuidados Intensivos Neonatal, Centro de Obstetricia y Ginecología, Santo Domingo, República Dominicana. ORCID: https://orcid.org/0000-0002-8492-7211, email: mendoza.em08@gmail.com
