Ciencia, Ambiente y Clima, Vol. 6, No. 2, julio-diciembre, 2023 ISSN (impreso): 2636-2317 • ISSN (en línea): 2636-2333 • Sitio web: https://revistas.intec.edu.do/
MECHANISMS OF ACTION OF FLAVONOIDS: ANTIOXIDANT, ANTIBACTERIAL AND ANTIFUNGAL PROPERTIES
Mecanismos de Acción de los Flavonoides: Propiedades Antioxidantes, Antibacterianas y Antifúngicas
DOI: https://doi.org/10.22206/cac.2023.v6i2.3021
BIANCA RODRÍGUEZ1, LIRIANNY PACHECO2, IRAN BERNAL3 Y MARIANNE PIÑA4
1 Instituto Tecnológico de Santo Domingo (INTEC), República Dominicana. ORCID: 0009-0002-4407-0345
Correo-e: bia.rodh@gmail.com
2 Instituto Tecnológico de Santo Domingo (INTEC), República Dominicana. ORCID: 0000-0002-9169-6597
Correo-e: liriannybieber@gmail.com
3 Instituto Tecnológico de Santo Domingo (INTEC), República Dominicana. ORCID: 0009-0000-4302-7260
Correo-e: Iranbernal@outlook.com
4 Instituto Tecnológico de Santo Domingo (INTEC), República Dominicana. ORCID: 0009-0001-7843-7523
Correo-e: 1087029@est.intec.edu.do
Recibido: 30/11/2023 • Aprobado: 28/12/2023
Cómo citar: Rodríguez, B., Pacheco, L., Bernal I. y Piña, M. (2023). Mechanisms of Action of Flavonoids: Antioxidant, Antibacterial and Antifungal Properties. Ciencia, Ambiente y Clima, 6(2), 33-66. https://doi.org/10.22206/cac.2023.v6i2.3021
Abstract
Flavonoids, a diverse group of natural polyphenolic compounds found in various sources like fruits and vegetables, have garnered attention for their diverse biological activities, including antioxidant, antibacterial, and antifungal properties. This comprehensive review delves into the mechanisms through which flavonoids combat oxidative stress and hinder the growth of bacteria and fungi. In terms of antioxidative action, flavonoids showcase their efficacy by neutralizing reactive oxygen species through hydrogen atom and electron donation, augmenting endogenous antioxidant enzyme activity, and chelating transition metal ions. Their antibacterial effects target both Gram-positive and Gram-negative bacteria, disrupting cell membranes, inhibiting key enzymatic processes, and suppressing efflux pumps, collectively impeding bacterial growth, and causing cell death. Additionally, flavonoids exhibit antifungal properties by interfering with fungal cell membrane integrity, disrupting ergosterol biosynthesis, and modulating critical signal transduction pathways, ultimately hindering fungal growth and pathogenicity. This nuanced understanding of flavonoid mechanisms not only holds promise for therapeutic development but also inspires future advancements in pharmacological research.
Keywords: Flavonoids; antibiotic resistance; antibacterial mechanisms; antioxidant mechanisms; antifungal activity.
Resumen
Los flavonoides, un grupo diverso de compuestos polifenólicos naturales que se encuentran en fuentes como frutas y verduras, han acaparado la atención por sus actividades biológicas, incluidas las propiedades antioxidantes, antibacterianas y antifúngicas. Esta exhaustiva revisión profundiza en los mecanismos en lo que los flavonoides combaten el estrés oxidativo y dificultan el crecimiento de bacterias y hongos. En cuanto a la acción antioxidante, los flavonoides muestran su eficacia neutralizando las especies reactivas del oxígenomediante la donación de átomos de hidrógeno y electrones, aumentando la actividad de enzimas antioxidantes endógenas y quelando iones de metales de transición. Sus efectos antibacterianos se dirigen a las bacterias Gram positivas y negativas, alterando las membranas celulares, inhibiendo procesos enzimáticos clave y suprimiendo las bombas de eflujo, impidiendo el crecimiento bacteriano y causando la muerte celular. Además, los flavonoides presentan propiedades antifúngicas al interferir en la integridad de la membrana celular fúngica, alterando la biosíntesis del ergosterol y modulando vías críticas de transducción de señales, impidiendo en última instancia el crecimiento y la patogenicidad de los hongos. Este conocimiento sobre los mecanismos de los flavonoides no sólo es prometedor para el desarrollo terapéutico, sino que también inspira futuros avances en la investigación farmacológica.
Palabras clave: Flavonoides; resistencia antibiótica; mecanismos antibacterianos; mecanismos antioxidantes; actividad antifúngica.
1. Introduction
Flavonoids are specialized secondary metabolites composed of a large group of polyphenolic compounds present abundantly in plants, fruits, vegetables, grains, and seeds, being responsible for color, fragrance, and flavor characteristics (Dias, Pinto, & Silva, 2021; Khan et al., 2021). Commonly they’re found in their glycoside form but can also exist in their free form. They are an important part of the human diet and provide antioxidant properties necessary for the correct development and control of biological systems including growth, genetic expression, and oxidative stress (OS) control (Crozier, Jaganath, & Clifford, 2009; Tsanova-Savova, Denev, & Ribarova, 2018). Approximately 10,000 different compounds have been reported considering them as the third most abundant bioactive chemical species in plants after terpenes and alkaloids (Dey et al., 2020; Ninkuu et al., 2021; Panche, Diwan, & Chandra, 2016).
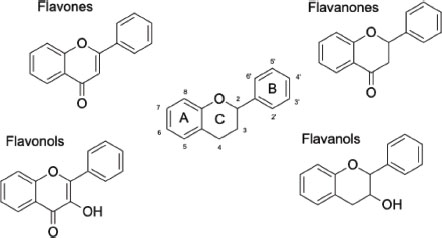
Flavonoids are classified into different categories depending on their structures and physicochemical properties. Chemically speaking, flavonoids are diphenylpropanes’ that follow the structure C6-C3-C6 where the aromatic ring linked to the pyran heterocyclic ring twice is identified as ring A, the lasting aromatic ring is identified as ring B and the pyran ring is C (Kejík et al., 2021; Speisky, Shahidi, Costa De Camargo, & Fuentes, 2022). Substituents and unsaturations divide flavonoids into different categories. Different patterns are present among flavonoid categories, for instance all flavones and flavonols possess an unsaturation between C2 and C3, while all flavones, flavonols, and flavanones possess a carbonyl substituent in C4 (Figure 1) (Dias et al., 2021; Heim, Tagliaferro, & Bobilya, 2002). Just as substituents and unsaturations confer flavonoids their classification, they’re the ones that confer their different properties. The presence or absence of different functional groups (i.e. hydroxyl, methoxyl, carbonyl, etc.) can provide flavonoids with radical scavenging properties and the capacity to chelate metals (Kejík et al., 2021; Martínez-Flórez, González-Gallego, & Culebras, 2002).
Figure 1
Flavonoid basic structure and principal categories structures
Bioavailability of flavonoids in complex biological systems is the main problem with the expression of all in vitro properties described in the literature. As mentioned, flavonoids are present in most plants and vegetables (Table 1) but, when ingested, their properties can be affected by the organism's metabolism (Liu et al., 2023; Williamson, Kay, & Crozier, 2018). In humans, flavonoids from dietary sources go through a two-step metabolism starting with de-glycosylation and other modifications such as functional group oxidation/addition in the small intestine. Later, they enter the liver through the portal vein and microsomal enzymes convert them into simpler forms. Finally, after hepatic first-pass metabolism, flavonoids reach and bind to their target sites (Khan et al., 2021). Later, metabolites in their hepatic second-pass are filtered out by the liver through urine. The first step of this pathway leads to changes in the structural composition of flavonoids that later result in changes to structural-related properties such as radical scavenging by resonance, etc (Kejík et al., 2021).
Table 1
Flavonoids major sources
Flavonoids |
Common compounds |
Major Source |
Reference |
Flavonols |
Quercetin Myricetin Kaempferol |
Onions, kales, lettuce, tomatoes, apples, grapes, broccoli |
(Khan et al., 2021; Tsanova-Savova et al., 2018; Waheed Janabi et al., 2019) |
Flavanols |
Catechins Epicatechins Gallocatechin |
Tea, cocoa, nectarine, pear, plum, mango, peach, raspberry |
(Khan et al., 2021; Tsanova-Savova et al., 2018; Waheed Janabi et al., 2019) |
Flavones |
Apigenin Luteolin Chrysin |
Citrus fruits, green tea, olive oil, parsley, peppermint, thyme |
(Khan et al., 2021; Tsanova-Savova et al., 2018; Waheed Janabi et al., 2019) |
Flavanones |
Eriodictyol Hesperetin Naringenin |
Orange, mandarin, lime, grapefruit, lemon |
|
Anthocyanidins |
Cyanidin Malvidin Petunidin Peonidin |
Berries, red cabbage, plums, purple corn, black beans |
(Kong, Chia, Goh, Chia, & Brouillard, 2003; Maisuria, Okshevsky, Déziel, & Tufenkji, 2019) |
Proanthocyanidins |
Catechins Polymers Epicatechins Polymers |
Red cabbage, broccoli, apple, grapes, berries |
However, bioavailability differs in biological systems simpler than the animal form. In prokaryotic and some simpler eukaryotic organisms, flavonoids have different properties and activities. In single-cell and some small pluricellular organisms, flavonoids bioavailability, metabolism and mechanisms of action are different given the fact that they may have different interactions with intrinsic metabolic pathways due to the lack of ‘control’ systems they possess (Yuan et al., 2021a). They can interact with DNA producing alteration and mutations, inhibit ATP-synthetase, lead to membrane disruption, and in some cases induce OS, contrary to the effect flavonoids portray in complex organisms such as plants and animals (Shamsudin et al., 2022a; Yuan et al., 2021a). These properties lead up to a broad list of opportunities and approaches to be taken into consideration when developing new technologies that apply flavonoids as their main compounds.
Beyond their well-documented antioxidant capabilities, flavonoids have been attributed with a broad spectrum of beneficial effects, including antibacterial and antifungal activities, making them versatile bioactive compounds with potential therapeutic applications. In this paper, we will delve into the intricate molecular mechanisms that underlie the multifaceted properties of flavonoids, shedding light on how they exert their beneficial effects in various physiological contexts.
2. Antioxidant Properties
In biological systems, OS indicates a condition that occurs when oxidizing substances (i.e. free radicals) accumulate and react resulting in consequences such as changes in the integrity of important macromolecules. Generally, in cells, an accumulation of both reactive oxygen species (ROS) and reactive nitrogen species (RNS) build up to the generation of OS. However, the increase in oxidative assaults on targeted biological macromolecules results in the malfunctioning of proteins, membranes, and nucleic acids, leading up to physiological consequences on the systems being injured including DNA mutation and genetic instability (Giorgio, 2015; Mucha, Skoczyńska, Małecka, Hikisz, & Budzisz, 2021).
ROS are responsible for the alteration, deletion, and accumulation of biological macromolecules over an organism’s lifespan. DNA deletions, specifically mitochondrial DNA, causes deterioration of cell function over time resulting in mutations and malignant proliferations (Mikhed, Daiber, & Steven, 2015). Also, DNA injuries can also promote ROS production in large quantities due to the generation of unstable compounds. In example, nucleotides degradation products by hydroxyl free radicals not only lead to DNA-protein cross-links (i.e. Gua-Lys), but also, they generate radical intermediates that follow up chain reactions that regenerate ROS (Cadet & Wagner, 2013). Following DNA modifications, OS main consequence in biological systems comes due to lipid peroxidation leading to cellular membrane damage and, consequently, changes in fluidity and permeability, alterations in active transport proteins and inhibition of some metabolic processes (Catalá & Díaz, 2016; Xian et al., 2021).
Flavonoids exhibit remarkable antioxidant properties by exerting their influence over OS in biological systems through a range of mechanisms. These mechanisms encompass both direct and indirect modes of oxidative control, and they contribute significantly to the protective effects of flavonoids within the body. Even though the main mechanisms by which flavonoids control OS remains unclear, recently, some studies have speculated how flavonoids control ROS and RNS concentrations in biological systems (Liang et al., 2014; Williams, Spencer, & Rice-Evans, 2004).
2.1. Free Radical Scavenging
One of the primary ways in which flavonoids act as antioxidants is by directly scavenging free radicals. Free radicals, such as ROS and RNS, are highly reactive molecules that can damage cellular components like proteins, lipids, and DNA. Flavonoids can neutralize these harmful free radicals, preventing them from causing cellular damage and OS (Liang et al., 2014). Flavonoid’s great ability is to stabilize the corresponding radicals due to the presence of unsaturation, electron-donating groups, and aromatic rings; they donate hydrogen radicals that stabilize ROS and RNS (Khan et al., 2021; Williams, Spencer, & Rice-Evans, 2004).
Most flavonoids can portray antioxidant activity due to their stable backbone structure. Nevertheless, substituents and their alteration by organism’s metabolism play a very important role in free radical scavenging properties of flavonoids (Dias et al., 2021). Main chemical criteria required for flavonoids to scavenge free radicals and stabilize their structures are:
• -OH substituents in positions 2’ and 6’ in ring B (Heim et al., 2002).
• -OH substituents in positions 3 and 5 with C=O in C4 (Martínez-Flórez et al., 2002).
• Double bond between C2 and C3 with C=O in C4 (Baldim et al., 2017).
Considering these criteria, flavonols (C2-C3 double bond, C3 -OH, C4=O) should exert the most powerful antioxidant properties due to their basic structures, despite that, literature describes flavones (C2-C3 double bond, C3 -OH, C4=O) as better for protecting the body against oxidative species (Panche et al., 2016). In vitro flavonols may exert better properties than flavones, yet in vivo the story is different.
Although metabolism affects the integrity of flavonoids and their properties, it has been noted that, in some cases, oxidative metabolism in their phenolic moieties (commonly found in the addition of -OH substituents) leads to better antioxidant properties (Speisky et al., 2022). Probably, oxidation of flavones is what leads to them having better antioxidant properties than flavonols in vivo, still they may also go through metabolic pathways that affect their structures and substituents.
Flavonoids can also interfere with inducible nitric-oxide (NO) synthesis activity. NO is a signaling molecule produced by different cells in biological systems with major physiological functions including vasodilation, neurotransmission, immune function, among others (Tejero, Shiva, & Gladwin, 2019), its production is tightly controlled in cells given the fact that they may react with free radicals present in the cell and produce damaging peroxynitrite. Under these circumstances, flavonoids are used as antioxidants scavenging free radicals that can no longer react with NO producing peroxynitrite (Nijveldt et al., 2001).
2.2. Interaction with Metal Ions
Flavonoids also display metal-chelating properties. Transition metals like iron and copper can participate in the Fenton reaction, generating highly reactive hydroxyl radicals that later contribute to lipid peroxidation (Beaufay et al., 2020). Some flavonoids can chelate these metal ions, rendering them inactive and thereby inhibiting the formation of destructive hydroxyl radicals. This metal-chelating action contributes to the antioxidant defense provided by flavonoids.
So far, interaction with ions is poorly described. Still, some detailed studies have described chelates by analyzing UV-Vis absorption spectra using Electrospray Ionization Mass Spectrometry (ESI-MS). It has been found that flavonoids have affinity to iron and copper ions and reduce them creating chemical complexes (Cherrak et al., 2016). Metal interactions depend on flavonoid structures; all flavonoids have shown high reducing capacity for copper ions, but only flavones for iron ions (Edo et al., 2023; Mira et al., 2002).
While considerable research has been conducted on flavonoids and their interactions with metal ions, it's important to note that there is still a substantial amount of investigation to be undertaken in this field. Flavonoids' ability to chelate metal ions and mitigate OS is well-established (Horniblow, Henesy, Iqbal, & Tselepis, 2017; Kejík et al., 2021), but the full extent of their in vivo potential applications and the nuances of these interactions remain subjects of ongoing exploration. The precise mechanisms, the specificity of flavonoids for different metal ions, and the implications of these interactions in various biological contexts continue to be areas of active investigation (Mucha et al., 2021). Further research in this domain holds promise for uncovering novel therapeutic avenues and enhancing our understanding of the multifaceted roles that flavonoids can play in living systems.
2.3. Gene Expression
Flavonoids’ main indirect action mechanism for OS in biological systems is through intercellular antioxidant signaling pathways that induce the production of endogenous antioxidants (Khan et al., 2021). In vitro studies have consistently demonstrated that the antioxidant properties of flavonoids primarily operate through the regulation of gene expression. Flavonoids exhibit their antioxidant potential by modulating the expression of key genes involved in cellular antioxidant defense mechanisms. These compounds are known to upregulate the expression of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), which collectively scavenge ROS and reduce OS (He, Ru, & Wen, 2020). Additionally, flavonoids can influence the expression of genes associated with inflammation, apoptosis, and other pathways related to oxidative damage, further emphasizing their gene-centric mechanism in conferring antioxidant protection in vitro.
Keap1-Nrf2-ARE is one of the most important cellular mechanisms to produce endogenous antioxidants during OS conditions. It consists of 3 major cellular components: Kelch-like ECH-associated protein 1 (Keap1), nuclear factor erythroid 2-related factor 2 (Nrf2) and antioxidant response element (ARE) (Johnson et al., 2008; Lee & Hu, 2020). Keap1 functions like a sensor for OS and a negative regulator of Nrf2 by binding to it impeding its antioxidant response. In addition, when Keap1 binds to Nrf2 it stimulates the spontaneous degradation of Nrf2 protein by ubiquitination. When Nrf2-Keap dissociation occurs Nrf2 is translocated to the nucleus where it activates downstream proteins that directly influence up-regulation of antioxidant genes (Baird & Yamamoto, 2020; Satta, Mahmoud, Wilkinson, Yvonne Alexander, & White, 2017). This dissociation occurs when some residues in Keap1 are oxidized leading to the release of Nrf2 moiety, decreasing Nrf2 ubiquitination and increasing its nuclear translocation and activation (Lo, Li, Henzl, Beamer, & Hannink, 2006; Silva-Islas & Maldonado, 2018).
As mentioned, flavonoids can affect endogenous antioxidant synthesis pathways. These metabolites induce conformational changes in Keap1 influencing the activation of the Nrf2/ARE pathway in the absence of oxidative inducers, leading to the subsequent activation of “antioxidant genes” like GPx, SOD, and CAT (Golonko, Olichwier, Swislocka, Szczerbinski, & Lewandowski, 2022; Suraweera, Rupasinghe, Dellaire, & Xu, 2020; Silva-Islas & Maldonado, 2018). An early activation of the Nrf2 pathway in response to low levels of specific ROS and electrophiles could serve as a critical defense mechanism for cells. Activation by flavonoids could not only safeguards cells from the potentially harmful consequences of prolonged ROS accumulation, which would otherwise lead to OS and redox imbalance, but it could also play a pivotal role in preventing the covalent attachment of electrophilic compounds to DNA and specific proteins essential for cellular functions (Speisky et al., 2022).
3. Antibacterial Properties
Antibiotic resistance is the phenomenon defined as the ability of microorganisms to develop a physiological mechanism to combat the effect of drugs so that, instead of dying, they continue to proliferate (Aljeldah, 2022). Resistance occurs in bacteria when the latent resistance gene is activated because of antibiotic overuse. The microorganism will activate a mechanism of action to evade the bactericidal activity of the antibiotic by different biochemical pathways, the main ones being based on inactivating the drug, reducing the effectiveness of the drug, altering the target site of the antibiotic, modifying the drug, bypassing the antibiotic target by producing alternative proteins and activating efflux pumps (Pulingam et al., 2022). Therefore, the indiscriminate and abusive use of antibiotics is conducive to the generation of bacterial resistance against drugs (Morrison & Zembower, 2020). This continuous increase in resistant strains makes it necessary to promote the development of new compounds that can be used in an environment of increasing multi-resistance.
Flavonoids have demonstrated important properties against plant pathogens, which could be effectively applied to fight human pathogens. The potential factors contributing to the antibacterial nature of these compounds might be ascribed to mechanisms different from conventional drugs such as disruption of membrane integrity, interference in efflux pumps, nucleic acid synthesis, and coenzyme metabolism (Donadio et al., 2021; Górniak, Bartoszewski, & Króliczewski, 2019; Osonga et al., 2019). Some other principal mechanisms are shown in Figure 2.
Figure 2
Main action mechanisms of flavonoids antibacterial activity
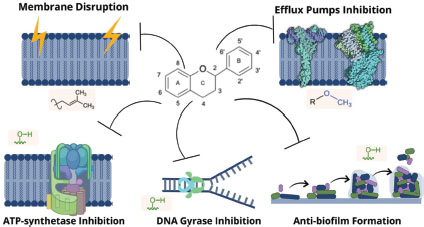
Source: Modified from Górniak et al., 2019.
3.1. Disruption of Cell Membranes
The membrane of bacteria is of high importance for bacterial survival and has processes that guarantee its integrity; any damage can lead to metabolic dysfunctions and bacterial death. Different studies support the theory that the relationship between antibacterial activity and membrane interference proceeds to reduce bacterial membrane fluidity (Sarbu, Bahrin, Babii, Stefan, & Birsa, 2019; Thebti et al., 2023). Bacteria susceptibility to antibacterial drugs has different effects on Gram-positive and Gram-negative bacteria due to differences in the cell membrane. Gram-positive bacteria feature a thick cell wall forming a substantial peptidoglycan layer (Nakamura et al., 2015). In contrast, Gram-negative bacteria possess an outer membrane consisting of structures like lipopolysaccharides, phospholipids, and a thinner layer of peptidoglycan (Osonga et al., 2019). This unique structure contributes to the Gram-negative cell wall's increased complexity and serves as a nearly impermeable barrier, limiting the entry of foreign molecules. In contrast, Gram-positive bacteria possess only peptidoglycans in their cell wall, making them more permeable and thus rendering them more sensitive to external influences. Consequently, Gram-positive bacteria are more susceptible, and flavonoids are found to have higher efficacy against Gram-positive bacteria than that of Gram-negative (Donadio et al., 2021; Goyal, Aggarwal, & Garg, 2010).
Research findings imply that the primary site where flavonoids exert their effects on Gram-positive bacteria is likely the cell membrane. This action probably involves mechanisms such as damaging phospholipid bilayers, inhibiting the respiratory chain, or disrupting ATP synthesis (Yuan et al., 2021). Different studies have used regression equations to calculate the antibacterial activities of plant flavonoids towards Gram-positive bacteria consisting of the physicochemical parameter ACD/LogP or LogD7.40 and the minimum inhibitory concentration (MIC, an indicator of antibacterial activity). LogP is a measure of the partitioning of a compound between a nonpolar (lipid-rich) and a polar (water-rich) environment. It expresses lipophilicity which is a critical factor in determining how easily a substance can pass through cell membranes (Mälkiä, Murtomäki, Urtti, & Kontturi, 2004). The LogD of flavonoids will change with the pH of the environment. As the pH increases, the LogD will correspondingly decrease. This is because, at higher pH levels, more of the flavonoid molecules will exist in their ionized (charged) form, which is less lipophilic. The success or failure of antibacterial agents to reach their target depends on their lipophilicity properties and how they interact with the cell membrane (Farhadi, Khameneh, Iranshahi, & Iranshahy, 2019). Researchers indicate that the relationship between the antibacterial activity of flavonoids and lipophilicity is not linear but rather it appears to have a curved shape. In this context, it suggests that as the lipophilicity (LogP or LogD7.40) of flavonoids increases, the antibacterial activity does not increase in a linear fashion but instead reaches a point where further increases in lipophilicity has diminishing returns or even adverse effects. This indicates that the lipophilicity of flavonoids is a key factor for their inhibitory activities to Gram-positive bacteria (Yuan et al., 2021).
Lipophilicity depends mostly on factors such as pH environment and molecular structures. Moderate to high lipophilicity plays a crucial role in enhancing the bioactivity of flavonoids facilitating more effective interactions or absorption within biomembranes (Thebti et al., 2023). Mechanisms involved in the interaction of flavonoids with lipid bilayer include the interaction at the membrane interface, where the polar heads of phospholipids meet the more hydrophilic flavonoids (Yuan et al., 2021). Within Gram-positive bacteria, the uptake of bioactive compounds primarily relies on hydrophobic interactions with the peptidoglycan, thereby facilitating the attachment and absorption of flavonoids (Donadio et al., 2021). In relation to structural activity, results from the regression equation show that the inhibitory effects of flavonoids on Gram-positive bacteria are enhanced when alkyl groups, particularly isopentyl, are incorporated into the flavonoid structures. This effect is observed regardless of the specific carbon position where the alkyl group is introduced (Shamsudin et al., 2022). This enhancement can be understood as follows: the addition of alkyl groups increases the lipophilicity or LogP of the flavonoids, thereby promoting their interactions with the phospholipids in cell membranes. However, it is important to note that an excessive introduction of alkyl groups can significantly elevate the LogP values of these flavonoids, making them excessively lipophilic and unable to pass through the hydrophilic region of phospholipid bilayers. However, the LogP value diminishes when polar groups, such as hydroxyl and glycosyl, are added to their chemical structures. This can be understood as an excessive increase in hydrophilicity due to the double bond, which hampers the flavonoids’ ability to penetrate phospholipid bilayers and interact with the hydrophobic regions of cell membranes (Yuan et al., 2021, 2022). Osorio et al. (2021) confirmed that the antibacterial efficacy of flavonoids in Staphylococcus aureus seems to be influenced by the saturation of the double bond at the C2–C3 position and the presence of hydroxyl substituents. According to Shamsudin et al., (2022), the quantity of OH groups did not hold significance, but the positioning of two hydroxyl groups in ring A and the absence of any hydroxylation in ring B within the compound were emphasized as factors influencing potent antibacterial activity. In this study, flavones showed higher bacterial inhibition against Gram-positive bacteria (Shamsudin et al., 2022).
Moreover, the presence of the free phenol hydroxyl group at C7 in the flavonoid chalcone plays a role in the antibacterial activity against Gram-positive bacteria as shown in Figure 3. Furthermore, halogenated compounds are the most active molecule against Gram-positive bacteria (Shamsudin et al., 2022; Thebti et al., 2023). The inclusion of electron-withdrawing groups was regarded as a beneficial factor for antibacterial effectiveness since they possess the ability to accept hydrogen bonds. This ability to accept electrons allows them to form covalent bonds with other atoms or groups such as hydrogen bonds. Shoaib et al. (2020) results confirmed how halogenation at C6 of ring A promotes the antibacterial action for halogenated flavones as shown in Figure 4 (Shoaib et al., 2020).
Figure 3
Structural activity relationship of chalcones in Gram-positive bacteria
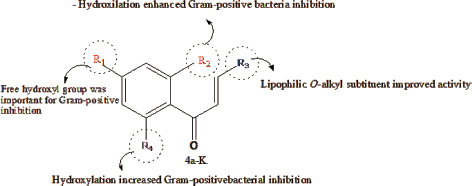
Source: Modified from Shamsudin et al., 2022.
Figure 4
Structural activity relationship of halogenated flavone in Gram-positive bacteria
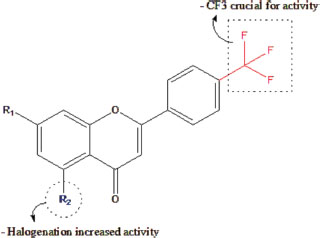
Source: Modified from Shamsudin et al., 2022.
Regarding Gram-negative bacteria, flavonoids possess a structure that interacts with lipopolysaccharide membranes through mechanisms that involve partitioning into the hydrophobic core of the membrane and the formation of hydrogen bonds at the membrane interface. For example, catechins, especially epicatechins, have been studied for their effects in Gram-negative bacteria, and it has been found that they can damage the bacterial membrane and trigger the inactivation of enzymes both inside and outside the cell (Górniak et al., 2019; Xie, Yang, Tang, Chen, & Ren, 2014). In addition, it has been observed that certain catechins generate an oxidative explosion when interacting with bacteria, causing the production of ROS that alter membrane permeability and cause damage. However, these oxidative bursts only occur at high concentrations of certain catechins. Furthermore, Gram-negative bacteria have been found to be less susceptible to these effects due to their negatively charged outer membrane (Farhadi et al., 2019). Other flavonoids have also demonstrated membrane disruptive activities, such as quercetin, apigenin, and morin, which cause destabilization of membrane structure and leakage of intracellular substances. Nonetheless, non-specific interactions can induce structural changes in the membrane and affect the pharmacological properties of flavonoids (Górniak et al., 2019). The mechanism that forms this interaction between flavonoid and bacterial membrane remains. However, flavonoids have significant potential as antibacterial agents, but more research is required to fully understand their mechanisms of action and effectiveness against different types of bacteria (Górniak et al., 2019; Sanver, Murray, Sadeghpour, Rappolt, & Nelson, 2016).
3.2. Inhibition of Nucleic Acid Synthesis
The inhibition of nucleic acid synthesis by flavonoids refers to the ability of certain flavonoid compounds to disrupt or impede the process of DNA and RNA synthesis in bacteria mainly acting as a topoisomerase inhibitor. In Gram-positive bacteria, flavonoids such as quercetin and apigenin have been reported to inhibit DNA gyrase from Mycobacterium smegmatis and Mycobacterium tuberculosis, an enzyme essential for DNA replication and maintenance in bacteria. By binding to DNA gyrase, flavonoids can prevent it from carrying out its normal function, leading to the inhibition of DNA replication. This finding was corroborated through investigations involving various gyrase subunits, which demonstrated quercetin's interaction with the B subunit of gyrase (Ohemeng, Schwender, Fu, & Barrett, 1993). This interaction resulted in the obstruction of the ATP binding pocket due to the establishment of hydrogen bonds facilitated by the 5, 7, and 3' -OH groups of quercetin with the amino acid residues of DNA gyrase. The mechanism by which quercetin interacts with the ATP binding pocket of the gyrase involves the formation of hydrogen bonds. Chrysin and Kaempferol also use this mechanism (Donadio et al., 2021; Górniak et al., 2019).
In addition, flavonoids also inhibit the supercoiling of DNA by engaging in competitive interactions with the ATP binding site found on the DNA gyrase B subunit (GyrB). Because of this enhanced interaction, DNA gyrase has a better facility to introduce breaks in the DNA strands, a process known as DNA cleavage (Donadio et al., 2021; Górniak et al., 2019). Furthermore, an influential factor for the inhibition of the synthesis of DNA and RNA is the formation of hydrogen bonds or the intercalation of the B ring within flavonoids in Proteus vulgaris and S. aureus. This could potentially contribute to the stacking of nucleic acid bases (Cushnie & Lamb, 2005; Osonga et al., 2019).
Regarding Gram-negative bacteria, what is known of enzyme inhibition is quite simple. Helicases are widely distributed motor proteins that play a crucial role in separating and rearranging nucleic acid duplexes through the hydrolysis of ATP, making them essential for DNA replication. Recent studies have identified flavonoids, specifically flavones and flavonols, as potential targets for these proteins. For example, luteolin, a flavone, and related flavonols such as morin and myricetin can inhibit the activity of replicative helicases (DnaB and RecBCD) in Escherichia coli. Additionally, myricetin has demonstrated the ability to restrain the growth of Gram-negative bacteria and has been suggested as a potent inhibitor of various enzymes, including DNA and RNA polymerases, viral reverse transcriptases, and telomerases (Górniak et al., 2019).
3.3. Enzymes Inhibitory Activity of Flavonoids in Bacteria
Flavonoids can interact with and inhibit enzymes involved in critical cellular processes, such as energy production, and cell wall formation. By disrupting these essential enzymatic activities, flavonoids can impede bacterial growth, making them potential candidates for developing antibacterial agents. This inhibition can help combat bacterial infections and may contribute to the development of new antibiotics or treatments against antibiotic-resistant bacteria. Flavonoids can also inactivate enzymes directly involved in pathogenicity and mechanisms of antibiotic resistance (Osonga et al., 2019). This involves the targeting of enzymes responsible for transporting toxins and virulent factors, as well as those involved in the inactivation of antibiotics. Certain flavonoids hindered the activity of enzymes such as aminoglycoside modifying enzymes (AMEs), acetyltransferases (AACs), nucleotidyltransferases (ANTs), or phosphotransferases (APHs) (Donadio et al., 2021). For instance, Sortase A (SrtA), found in the bacterium S. aureus, plays a critical role in attaching certain surface proteins of the bacterium to its peptidoglycan layer (Oh et al., 2011). This attachment is important for Gram-positive bacterium’s capacity to display these surface proteins and for its virulence, or its propensity to cause disease in humans. In Gram-positive bacteria, flavonoids extracted from plants bind to the SrtA enzyme preventing it from carrying out its normal catalytic functions (Oh et al., 2011).
Besides, flavonoids interact with key enzymes that participate in various anabolic and catabolic pathways. These pathways include processes like the synthesis of fatty acids and sterols, the construction of cell wall cross-linking, the Krebs cycle, and glucose metabolism (Donadio et al., 2021). For example, the effects of α-mangostin and xanthone compounds from flavonoids in Gram-positive bacteria involve the inhibition of certain enzymes within the respiratory chain of the bacteria. This inhibition could disrupt the normal flow of electrons during energy production (L. Zhang et al., 2023). Flavonoids can also affect the quinone pool through non-enzyme mechanisms. These mechanisms could include alterations in electron transfer processes, changes in the electrical potential across the bacterial membrane (membrane potential), and the generation of ROS. All these processes are vital for the normal functioning of the bacterial cell (Donadio et al., 2021).
3.4. Efflux Pumps and Flavonoid Interaction
The alterations observed in membrane permeability are frequently accompanied by elevated expression levels of efflux pumps (EP) in both Gram-negative and Gram-positive bacteria. These efflux pumps can either be specific to certain substances or possess a wide-ranging substrate specificity, a trait commonly found in multidrug-resistant bacteria. This essential function enables bacteria to regulate their internal microenvironment, uphold membrane potential, and efficiently remove toxic compounds and signaling molecules, all of which contribute to their survival and adaptability in challenging environments (Donadio et al., 2021). Findings indicate that, compared to the other mechanisms, flavonoids function more as enhancers of efflux pump activity rather than inhibitors (Górniak et al., 2019). Certain flavonoids often lead to the enhancement of antibiotic efficacy restoring the susceptibility of bacteria to antibiotic treatments (Donadio et al., 2021).
According to Donadio et al. (2021), the ability of flavonoids to inhibit EP is closely associated with their pattern of substituents. Myricetin compound proved that a greater number of -OH substituents in the B ring enhance their effectiveness in inhibiting efflux pumps in Gram-positive bacteria, S. aureus. However, methoxylated flavonoids, such as tetramethylscutellarein, seem to modulate EP activity even more efficiently. This heightened efficacy may stem from the increased lipophilicity of these molecules, which is a result of the presence of methoxyl groups (Donadio et al., 2021; Stavri, Piddock, & Gibbons, 2007). This suggests that lipophilicity is a shared characteristic among numerous efflux pump inhibitors and appears to be essential in EP inhibition within Gram-positive bacteria.
For instance, the MFS efflux pumps are a type of protein pump found in the cell membranes of bacteria. Their function is to pump out various substances from the bacterial cell, including antibiotics. A specific MFS pump called NorA found in S. aureus can pump out fluoroquinolone antibiotics. NorA is believed to contribute to resistance against fluoroquinolone antibiotics in S. aureus. Even so, the flavone chrysoberyl as well as the flavonol aglycones chrysosplenetin and penduletin were detected to significantly decrease expression of NorA acting as an as efflux inhibitors due to a methoxyl group in the B-ring (Donadio et al., 2021; Waditzer & Bucar, 2021).
On the other hand, a recent study highlighted the role of cranberry proanthocyanidins in interfering with antibiotic resistance mechanisms in Gram-negative bacteria such as E. coli, Proteus mirabilis and Pseudomonas aeruginosa (L. Zhang et al., 2023). These bacteria are intrinsically resistant to several antibiotics, but, when cranberry proanthocyanidins were combined with tetracycline, it prevented the development of resistance, suggesting a possible natural remedy for infections of the urinary tract (Donadio et al., 2021; Maisuria et al., 2019). It was shown that their effectiveness was related to the activity of EP. An in silico docking analysis revealed the most likely interaction regions between these proanthocyanidins and efflux pump proteins, providing information on the binding mode and stability of the resulting complexes. This study represents an example of research on the interaction between components of efflux pumps and flavonoid compounds. The interaction of proanthocyanidins with EP could interfere with the preferred binding of potentiated antibiotics, resulting in inhibition of antibiotic efflux (Donadio et al., 2021). However, research on EP inhibition by flavonoids in Gram-negative bacteria, such as E. coli and P. aeruginosa, has been limited to date (Maisuria et al., 2019).
3.5. Biofilm Formation Inhibition
Biofilms are organized communities of microorganisms that produce an extracellular matrix and represent a bacterial survival strategy, protecting embedded cells from antibacterial threats and the host immune system. Biofilm formation involves three stages: surface adhesion, maturation, and dispersion. During maturation, bacteria produce extracellular polysaccharides (EPS) that facilitate surface adhesion and cell-to-cell cohesion. Dispersal of biofilm cells can occur through factors that disrupt self-produced EPS and other mechanisms, allowing cells to move toward the environment or hosts (Abdullahi, Igwenagu, Mu’azu, Aliyu, & Umar, 2016; Górniak et al., 2019; Jamal et al., 2018; Khan et al., 2021).
Additionally, efflux pump inhibitors (EPIs) influence the formation of biofilms. For instance, pinostrobin is a flavanone found in pine wood, it was observed to increase the permeability of bacterial membranes in both Gram-positive and Gram-negative bacteria such as Enterococcus faecalis, S. aureus, E. coli, and P. aeruginosa. This heightened membrane permeability was linked to its effectiveness as an EPI and ability to inhibit biofilm formation, particularly in Gram-negative bacteria. Interestingly, pinostrobin's mechanism for preventing biofilm formation was suggested to be different from its EPI action and might not involve the suppression of curli genes, which sets it apart from previous findings in Salmonella typhimurium (Christena et al., 2015; Górniak et al., 2019; Sana & Jameel, 2015). Similarly, tea derived EGCG (Epigallocatechin gallate) demonstrated effectiveness against both the free-floating and biofilm forms of E. faecalis. It not only hindered bacterial growth but also suppressed the activity of specific genes associated with biofilm formation. Additionally, certain prenylated flavonoids obtained from Epimedium plants disrupted the formation of Porphyromonas gingivalis biofilms. However, the precise mechanism by which these flavonoids counter biofilm formation remains unclear (Kariu et al., 2017).
4. Mechanisms of Action in Fungi
Flavonoids have valuable antifungal properties. Their presence in plants benefits survival and health by inhibiting the growth of harmful fungi and protecting against factors like UV radiation (De Conti Lourenço, da Silva Melo, & de Almeida, 2013). Flavonoids serve as chemical signals in the partnership between arbuscular mycorrhizal plants and fungi. When the plant’s roots release flavonoids, they act as messages that attract mycorrhizal fungi, facilitating a symbiotic relationship (Ghitti, Rolli, Crotti, & Borin, 2022). Plants can fend off pathogenic fungi by using compounds like flavonoids, which may act as antioxidants (Nabil-Adam et al., 2023), others as potent antimicrobials (Weston & Mathesius, 2013). Similarly, flavonoids have a versatile mechanism of action inhibiting fungal growth (Al Aboody & Mickymaray, 2020).
4.1. Inhibition of Cell Wall Formation
The wall of fungi is formed by compounds highlighting β-glucan and chitin, which are polysaccharides that provide integrity to the cell wall (Garcia-Rubio, de Oliveira, Rivera, & Trevijano-Contador, 2020). Glycyrrhiza glabra L belongs to the family of Fabaceae known as Leguminosae (Licorice), a plant that can be found in high temperate areas of southern Europe, India and Pakistan and has been grown experimentally in the United States and has been noted for its medicinal properties (El-Saber Batiha, Magdy Beshbishy, El-Mleeh, M. Abdel-Daim, & Prasad Devkota, 2020). Its roots have phenolic compounds, highlighting flavonoids such as glabridin and isoflavonoids. These flavonoids have ecophysiological functions playing a role in plant interaction (Simmler, Pauli, & Chen, 2013):
• Defensive agents against different types of organisms.
• Phytoalexins and isoflavonoids function as phytoalexins, substances produced by plants in response to fungal infection.
Glabridin has antifungal properties against several species of fungi, managing to alter the cell wall structure of these pathogens. Zhang et al., (2020) demonstrated how glabridin inhibits the growth of different fungi. In filamentous fungi the synergy of the antifungal nystatin with the combination of glabridin stops growth and overexpress genes associated with apoptosis as this promotes apoptosis inducing factors.
4.2. Relationship between Flavonoids and Antifungals in Efflux Pumps System
Some fungi have a pumping system that allows them to expel substances. This mechanism takes place through proteins found in cell membranes of the fungus. ATP-binding cassette, for instance, a family of efflux pump proteins composed of nucleotide binding domains (NBD) aim to break down ATP to transport substances out of the cell (Holmes et al., 2016). Activation of these efflux pumps leads to drug resistance and substances that can block the operation of the efflux pump resulting beneficial for the fungus (Kang, Fong, & Tsang, 2010). The flavone apigenin inhibits the growth of Candida albicans when used with miconazole, reducing the production of ergosterol and blocking the action of efflux pumps that expel drugs from fungus (Mangoyi, Midiwo, & Mukanganyama, 2015).
Combining chemicals from natural sources with antifungal drugs is an action that treats and prevents drug resistance, a new therapeutic approach since lower doses minimize the toxicity of drugs (Kim, Woo, & Lee, 2019). Chalcones have the potential of efflux pump in C. albicans, when these flavonoids are combined with fluconazole they cannot eliminate it, improving the effectiveness of the treatment (Wang et al., 2016). Moreover, Fluconazole-resistant Candida tropicalis treated with flavonoids and fluconazole exerts an effect on the membrane structure of the fungus making it more permeable. In addition, it leads to an increase in ROS levels that has a regulatory function in cells. For instance, accumulating hydroxyl radicals that contribute to cellular aging (Da Silva et al., 2014).
5. Conclusions and Future Perspectives
This review aimed to outline the recent advances in flavonoid mechanisms in antimicrobial and antioxidant properties. Considering the urgent need for the discovery of novel antimicrobial agents or enhancers to increase the efficacy of existing drugs, the exploration of flavonoids as potential candidates holds great promise. Significantly, the generally recognized nontoxic nature of most flavonoids due to their widespread occurrence in various plant-derived foods and beverages, accentuates their potential utility. Among their various pharmacological effects described previously, it is evidence that specific structural features in flavonoids play a vital role in imparting antibacterial and antioxidant effects. The different mechanisms are achieved by disrupting bacterial cell membranes, interfering with essential enzymes, or inhibiting the synthesis of important cellular components like DNA. On the other hand, flavonoids' antioxidant mechanisms stem from their capacity to neutralize harmful free radicals and protect cells from OS. Their multifaceted antioxidant mechanisms, including scavenging free radicals, chelating metal ions, and regenerating other antioxidants, make them crucial defenders against oxidative damage and potential contributors to the prevention of various chronic diseases.
Mechanisms provided in the present review may assist in the optimization and understanding of a lead compound’s activity, provide a focus for toxicological attention, and aid in the anticipation of bacterial resistance. Given the current situation concerning antimicrobial properties and the escalating challenge of pathogen resistance mechanisms, flavonoids represent a promising avenue for further research and potential therapeutic applications. In conclusion, flavonoid compounds present an expansive and relatively unexplored reservoir of therapeutic agents, necessitating further studies to comprehensively elucidate the underlying mechanisms and realize their full in vivo antibacterial potential.
References
Abdullahi, U. F., Igwenagu, E., Mu’azu, A., Aliyu, S., & Umar, M. I. (2016). Intrigues of biofilm: A perspective in veterinary medicine. Veterinary World, 9(1), 12–18. https://doi.org/10.14202/vetworld.2016.12-18
Al Aboody, M. S., & Mickymaray, S. (2020). Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics, 9(2), 45. https://doi.org/10.3390/antibiotics9020045
Aljeldah, M. M. (2022). Antimicrobial Resistance and Its Spread Is a Global Threat. Antibiotics, 11(8), 1082. https://doi.org/10.3390/antibiotics11081082
Baird, L., & Yamamoto, M. (2020). The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Molecular and Cellular Biology, 40(13), e00099-20. https://doi.org/10.1128/MCB.00099-20
Baldim, J. L., Alcântara, B. G. V. D., Domingos, O. D. S., Soares, M. G., Caldas, I. S., Novaes, R. D., … Chagas-Paula, D. A. (2017). The Correlation between Chemical Structures and Antioxidant, Prooxidant, and Antitrypanosomatid Properties of Flavonoids. Oxidative Medicine and Cellular Longevity, 2017, 1–12. https://doi.org/10.1155/2017/3789856
Beaufay, F., Quarles, E., Franz, A., Katamanin, O., Wholey, W.-Y., & Jakob, U. (2020). Polyphosphate Functions In Vivo as an Iron Chelator and Fenton Reaction Inhibitor. mBio, 11(4), e01017-20. https://doi.org/10.1128/mBio.01017-20
Cadet, J., & Wagner, J. R. (2013). DNA Base Damage by Reactive Oxygen Species, Oxidizing Agents, and UV Radiation. Cold Spring Harbor Perspectives in Biology, 5(2), a012559–a012559. https://doi.org/10.1101/cshperspect.a012559
Catalá, A., & Díaz, M. (2016). Impact of Lipid Peroxidation on the Physiology and Pathophysiology of Cell Membranes. Frontiers in Physiology, 7. https://doi.org/10.3389/fphys.2016.00423
Cherrak, S. A., Mokhtari-Soulimane, N., Berroukeche, F., Bensenane, B., Cherbonnel, A., Merzouk, H., & Elhabiri, M. (2016). In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation. PLOS ONE, 11(10), e0165575. https://doi.org/10.1371/journal.pone.0165575
Christena, L. R., Subramaniam, S., Vidhyalakshmi, M., Mahadevan, V., Sivasubramanian, A., & Nagarajan, S. (2015). Dual role of pinostrobin-a flavonoid nutraceutical as an efflux pump inhibitor and antibiofilm agent to mitigate food borne pathogens. RSC Advances, 5(76), 61881–61887. https://doi.org/10.1039/C5RA07165H
Crozier, A., Jaganath, I. B., & Clifford, M. N. (2009). Dietary phenolics: Chemistry, bioavailability and effects on health. Natural Product Reports, 26(8), 1001. https://doi.org/10.1039/b802662a
Cushnie, T. P. T., & Lamb, A. J. (2005). Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents, 26(5), 343–356. https://doi.org/10.1016/j.ijantimicag.2005.09.002
Da Silva, C. R., De Andrade Neto, J. B., De Sousa Campos, R., Figueiredo, N. S., Sampaio, L. S., Magalhães, H. I. F., … Nobre Júnior, H. V. (2014). Synergistic Effect of the Flavonoid Catechin, Quercetin, or Epigallocatechin Gallate with Fluconazole Induces Apoptosis in Candida tropicalis Resistant to Fluconazole. Antimicrobial Agents and Chemotherapy, 58(3), 1468–1478. https://doi.org/10.1128/AAC.00651-13
De Conti Lourenço, R. M., da Silva Melo, P., & de Almeida, A. B. A. (2013). Flavonoids as Antifungal Agents. In M. Razzaghi-Abyaneh & M. Rai (Eds.), Antifungal Metabolites from Plants (pp. 283–300). Berlin, Heidelberg: Springer. Retrieved from https://doi.org/10.1007/978-3-642-38076-1_10
Dey, P., Kundu, A., Kumar, A., Gupta, M., Lee, B. M., Bhakta, T., … Kim, H. S. (2020). Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent Advances in Natural Products Analysis (pp. 505–567). Elsevier. Retrieved from https://linkinghub.elsevier.com/retrieve/pii/B9780128164556000159
Dias, M. C., Pinto, D. C. G. A., & Silva, A. M. S. (2021). Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules, 26(17), 5377. https://doi.org/10.3390/molecules26175377
Donadio, G., Mensitieri, F., Santoro, V., Parisi, V., Bellone, M. L., De Tommasi, N., … Dal Piaz, F. (2021). Interactions with Microbial Proteins Driving the Antibacterial Activity of Flavonoids. Pharmaceutics, 13(5), 660. https://doi.org/10.3390/pharmaceutics13050660
Edo, G. I., Ugbune, U., Onoharigho, F. O., Ezekiel, G. O., Ugbuwe, E., & Agbo, J. J. (2023). Investigation of the metal complexes and bioactive compound formed by coordination of bioactive phytochemical from ginger (Zingiber officinale) extracts to metal ions. Food Chemistry Advances, 3, 100337. https://doi.org/10.1016/j.focha.2023.100337
El-Saber Batiha, G., Magdy Beshbishy, A., El-Mleeh, A., M. Abdel-Daim, M., & Prasad Devkota, H. (2020). Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules, 10(3), 352. https://doi.org/10.3390/biom10030352
Farhadi, F., Khameneh, B., Iranshahi, M., & Iranshahy, M. (2019). Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytotherapy Research: PTR, 33(1), 13–40. https://doi.org/10.1002/ptr.6208
Garcia-Rubio, R., de Oliveira, H. C., Rivera, J., & Trevijano-Contador, N. (2020). The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Frontiers in Microbiology, 10. Retrieved from https://www.frontiersin.org/articles/10.3389/fmicb.2019.02993
Ghitti, E., Rolli, E., Crotti, E., & Borin, S. (2022). Flavonoids Are Intra- and Inter-Kingdom Modulator Signals. Microorganisms, 10(12), 2479. https://doi.org/10.3390/microorganisms10122479
Giorgio, M. (2015). Oxidative stress and the unfulfilled promises of antioxidant agents. Ecancermedicalscience, 9. https://doi.org/10.3332/ecancer.2015.556
Golonko, A., Olichwier, A. J., Swislocka, R., Szczerbinski, L., & Lewandowski, W. (2022). Why Do Dietary Flavonoids Have a Promising Effect as Enhancers of Anthracyclines? Hydroxyl Substituents, Bioavailability and Biological Activity. International Journal of Molecular Sciences, 24(1), 391. https://doi.org/10.3390/ijms24010391
Górniak, I., Bartoszewski, R., & Króliczewski, J. (2019). Comprehensive review of antimicrobial activities of plant flavonoids. Phytochemistry Reviews, 18(1), 241–272. https://doi.org/10.1007/s11101-018-9591-z
Goyal, P., Aggarwal, B. K., & Garg, S. (2010). A Study on Combinatorial Effects of Various Flavonoids for Their Antibacterial Potential Against Clinically Significant Bacterial Species. Hacettepe Journal of Biology and Chemistry, 38(4), 255–258. Retrieved from https://dergipark.org.tr/en/pub/hjbc/issue/61874/925966
He, F., Ru, X., & Wen, T. (2020). NRF2, a Transcription Factor for Stress Response and Beyond. International Journal of Molecular Sciences, 21(13), 4777. https://doi.org/10.3390/ijms21134777
Heim, K. E., Tagliaferro, A. R., & Bobilya, D. J. (2002). Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. The Journal of Nutritional Biochemistry, 13(10), 572–584. https://doi.org/10.1016/S0955-2863(02)00208-5
Holmes, A. R., Cardno, T. S., Strouse, J. J., Ivnitski-Steele, I., Keniya, M. V., Lackovic, K., … Cannon, R. D. (2016). Targeting efflux pumps to overcome antifungal drug resistance. Future Medicinal Chemistry, 8(12), 1485–1501. https://doi.org/10.4155/fmc-2016-0050
Horniblow, R. D., Henesy, D., Iqbal, T. H., & Tselepis, C. (2017). Modulation of iron transport, metabolism and reactive oxygen status by quercetin–iron complexes in vitro. Molecular Nutrition & Food Research, 61(3), 1600692. https://doi.org/10.1002/mnfr.201600692
Jamal, M., Ahmad, W., Andleeb, S., Jalil, F., Imran, M., Nawaz, M. A., … Kamil, M. A. (2018). Bacterial biofilm and associated infections. Journal of the Chinese Medical Association: JCMA, 81(1), 7–11. https://doi.org/10.1016/j.jcma.2017.07.012
Johnson, J. A., Johnson, D. A., Kraft, A. D., Calkins, M. J., Jakel, R. J., Vargas, M. R., & Chen, P. (2008). The Nrf2–ARE Pathway: An Indicator and Modulator of Oxidative Stress in Neurodegeneration. Annals of the New York Academy of Sciences, 1147(1), 61–69. https://doi.org/10.1196/annals.1427.036
Kang, K., Fong, W.-P., & Tsang, P. W.-K. (2010). Novel antifungal activity of purpurin against Candida species in vitro. Medical Mycology, 48(7), 904–911. https://doi.org/10.3109/13693781003739351
Kariu, T., Nakao, R., Ikeda, T., Nakashima, K., Potempa, J., & Imamura, T. (2017). Inhibition of gingipains and Porphyromonas gingivalis growth and biofilm formation by prenyl flavonoids. Journal of Periodontal Research, 52(1), 89–96. https://doi.org/10.1111/jre.12372
Kejík, Z., Kaplánek, R., Masařík, M., Babula, P., Matkowski, A., Filipenský, P., … Jakubek, M. (2021). Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond. International Journal of Molecular Sciences, 22(2), 646. https://doi.org/10.3390/ijms22020646
Khan, J., Deb, P. K., Priya, S., Medina, K. D., Devi, R., Walode, S. G., & Rudrapal, M. (2021). Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules, 26(13), 4021. https://doi.org/10.3390/molecules26134021
Kim, S., Woo, E.-R., & Lee, D. G. (2019). Synergistic Antifungal Activity of Isoquercitrin: Apoptosis and Membrane Permeabilization Related to Reactive Oxygen Species in Candida albicans. IUBMB Life, 71(2), 283–292. https://doi.org/10.1002/iub.1973
Kong, J.-M., Chia, L.-S., Goh, N.-K., Chia, T.-F., & Brouillard, R. (2003). Analysis and biological activities of anthocyanins. Phytochemistry, 64(5), 923–933. https://doi.org/10.1016/S0031-9422(03)00438-2
Lee, S., & Hu, L. (2020). Nrf2 activation through the inhibition of Keap1–Nrf2 protein–protein interaction. Medicinal Chemistry Research, 29(5), 846–867. https://doi.org/10.1007/s00044-020-02539-y
Liang, C., Chang, C., Liang, C., Hung, K., & Hsieh, C. (2014). In Vitro Antioxidant Activities, Free Radical Scavenging Capacity, and Tyrosinase Inhibitory of Flavonoid Compounds and Ferulic Acid from Spiranthes sinensis (Pers.) Ames. Molecules, 19(4), 4681–4694. https://doi.org/10.3390/molecules19044681
Liu, M., Zhang, G., Zhou, K., Wen, J., Zheng, F., Sun, L., & Ren, X. (2023). Structural characterization, antioxidant activity, and the effects of Codonopsis pilosula polysaccharides on the solubility and stability of flavonoids. Journal of Pharmaceutical and Biomedical Analysis, 229, 115368. https://doi.org/10.1016/j.jpba.2023.115368
Lo, S.-C., Li, X., Henzl, M. T., Beamer, L. J., & Hannink, M. (2006). Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. The EMBO Journal, 25(15), 3605–3617. https://doi.org/10.1038/sj.emboj.7601243
Maisuria, V. B., Okshevsky, M., Déziel, E., & Tufenkji, N. (2019). Proanthocyanidin Interferes with Intrinsic Antibiotic Resistance Mechanisms of Gram-Negative Bacteria. Advanced Science, 6(15), 1802333. https://doi.org/10.1002/advs.201802333
Mälkiä, A., Murtomäki, L., Urtti, A., & Kontturi, K. (2004). Drug permeation in biomembranes: In vitro and in silico prediction and influence of physicochemical properties. European Journal of Pharmaceutical Sciences, 23(1), 13–47. https://doi.org/10.1016/j.ejps.2004.05.009
Mangoyi, R., Midiwo, J., & Mukanganyama, S. (2015). Isolation and characterization of an antifungal compound 5-hydroxy-7,4’-dimethoxyflavone from Combretum zeyheri. BMC Complementary and Alternative Medicine, 15(1), 405. https://doi.org/10.1186/s12906-015-0934-7
Martínez-Flórez, S., González-Gallego, J., & Culebras, J. M. (2002). Los flavonoides: Propiedades y acciones antioxidantes. Nutr. Hosp.
Mikhed, Y., Daiber, A., & Steven, S. (2015). Mitochondrial Oxidative Stress, Mitochondrial DNA Damage and Their Role in Age-Related Vascular Dysfunction. International Journal of Molecular Sciences, 16(7), 15918–15953. https://doi.org/10.3390/ijms160715918
Mira, L., Tereza Fernandez, M., Santos, M., Rocha, R., Helena Florêncio, M., & Jennings, K. R. (2002). Interactions of Flavonoids with Iron and Copper Ions: A Mechanism for their Antioxidant Activity. Free Radical Research, 36(11), 1199–1208. https://doi.org/10.1080/1071576021000016463
Morrison, L., & Zembower, T. R. (2020). Antimicrobial Resistance. Gastrointestinal Endoscopy Clinics of North America, 30(4), 619–635. https://doi.org/10.1016/j.giec.2020.06.004
Mucha, P., Skoczyńska, A., Małecka, M., Hikisz, P., & Budzisz, E. (2021). Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules, 26(16), 4886. https://doi.org/10.3390/molecules26164886
Nabil-Adam, A., Elnosary, M. E., Ashour, M. L., El-Moneam, N. M. A., Shreadah, M. A., Nabil-Adam, A., … Shreadah, M. A. (2023). Flavonoids Biosynthesis in Plants as a Defense Mechanism: Role and Function Concerning Pharmacodynamics and Pharmacokinetic Properties. In Flavonoid Metabolism—Recent Advances and Applications in Crop Breeding. IntechOpen. Retrieved from https://www.intechopen.com/chapters/85627
Nakamura, K., Ishiyama, K., Sheng, H., Ikai, H., Kanno, T., & Niwano, Y. (2015). Bactericidal Activity and Mechanism of Photoirradiated Polyphenols against Gram-Positive and -Negative Bacteria. Retrieved from 10.1021/jf5058588
Nijveldt, R. J., Van Nood, E., Van Hoorn, D. E., Boelens, P. G., Van Norren, K., & Van Leeuwen, P. A. (2001). Flavonoids: A review of probable mechanisms of action and potential applications. The American Journal of Clinical Nutrition, 74(4), 418–425. https://doi.org/10.1093/ajcn/74.4.418
Ninkuu, V., Zhang, L., Yan, J., Fu, Z., Yang, T., & Zeng, H. (2021). Biochemistry of Terpenes and Recent Advances in Plant Protection. International Journal of Molecular Sciences, 22(11), 5710. https://doi.org/10.3390/ijms22115710
Oh, I., Yang, W.-Y., Chung, S.-C., Kim, T.-Y., Oh, K.-B., & Shin, J. (2011). In vitro sortase a inhibitory and antimicrobial activity of flavonoids isolated from the roots of Sophora flavescens. Archives of Pharmacal Research, 34(2), 217–222. https://doi.org/10.1007/s12272-011-0206-0
Ohemeng, K. A., Schwender, C. F., Fu, K. P., & Barrett, J. F. (1993). DNA Gyrase Inhibitory And Antibacterial Activity Of Some Flavones.
Osonga, F. J., Akgul, A., Miller, R. M., Eshun, G. B., Yazgan, I., Akgul, A., & Sadik, O. A. (2019). Antimicrobial Activity of a New Class of Phosphorylated and Modified Flavonoids. ACS Omega, 4(7), 12865–12871. https://doi.org/10.1021/acsomega.9b00077
Osorio, M., Carvajal, M., Vergara, A., Butassi, E., Zacchino, S., Mascayano, C., … Vásquez-Martínez, Y. (2021). Prenylated Flavonoids with Potential Antimicrobial Activity: Synthesis, Biological Activity, and In Silico Study. International Journal of Molecular Sciences, 22(11), 5472. https://doi.org/10.3390/ijms22115472
Panche, A. N., Diwan, A. D., & Chandra, S. R. (2016). Flavonoids: An overview. Journal of Nutritional Science, 5, e47. https://doi.org/10.1017/jns.2016.41
Pulingam, T., Parumasivam, T., Gazzali, A. M., Sulaiman, A. M., Chee, J. Y., Lakshmanan, M., … Sudesh, K. (2022). Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. European Journal of Pharmaceutical Sciences, 170, 106103. https://doi.org/10.1016/j.ejps.2021.106103
Sana, M., & Jameel, H. (2015). Miracle Remedy: Inhibition of Bacterial Efflux Pumps by Natural Products. Journal of Infectious Diseases & Therapy, 03(02). https://doi.org/10.4172/2332-0877.1000213
Sanver, D., Murray, B. S., Sadeghpour, A., Rappolt, M., & Nelson, A. L. (2016). Experimental Modeling of Flavonoid–Biomembrane Interactions. Langmuir, 32(49), 13234–13243. https://doi.org/10.1021/acs.langmuir.6b02219
Sarbu, L. G., Bahrin, L. G., Babii, C., Stefan, M., & Birsa, M. L. (2019). Synthetic flavonoids with antimicrobial activity: A review. Journal of Applied Microbiology, 127(5), 1282–1290. https://doi.org/10.1111/jam.14271
Satta, S., Mahmoud, A. M., Wilkinson, F. L., Yvonne Alexander, M., & White, S. J. (2017). The Role of Nrf2 in Cardiovascular Function and Disease. Oxidative Medicine and Cellular Longevity, 2017, 1–18. https://doi.org/10.1155/2017/9237263
Shamsudin, N. F., Ahmed, Q. U., Mahmood, S., Ali Shah, S. A., Khatib, A., Mukhtar, S., … Zakaria, Z. A. (2022). Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules, 27(4), 1149. https://doi.org/10.3390/molecules27041149
Shoaib, M., Ali Shah, S. W., Ali, N., Umar, N., Shah, I., Ullah, S., & Tahir, M. N. (2020). Synthesis, crystal studies and biological evaluation of flavone derivatives. Pakistan Journal of Pharmaceutical Sciences, 33(1), 11–20.
Silva-Islas, C. A., & Maldonado, P. D. (2018). Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacological Research, 134, 92–99. https://doi.org/10.1016/j.phrs.2018.06.013
Simmler, C., Pauli, G. F., & Chen, S.-N. (2013). Phytochemistry and biological properties of glabridin. Fitoterapia, 90, 160–184. https://doi.org/10.1016/j.fitote.2013.07.003
Speisky, H., Shahidi, F., Costa De Camargo, A., & Fuentes, J. (2022). Revisiting the Oxidation of Flavonoids: Loss, Conservation or Enhancement of Their Antioxidant Properties. Antioxidants, 11(1), 133. https://doi.org/10.3390/antiox11010133
Stavri, M., Piddock, L. J. V., & Gibbons, S. (2007). Bacterial efflux pump inhibitors from natural sources. Journal of Antimicrobial Chemotherapy, 59(6), 1247–1260. https://doi.org/10.1093/jac/dkl460
Suraweera, T. L., Rupasinghe, H. P. V., Dellaire, G., & Xu, Z. (2020). Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants, 9(10), 973. https://doi.org/10.3390/antiox9100973
Tejero, J., Shiva, S., & Gladwin, M. T. (2019). Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiological Reviews, 99(1), 311–379. https://doi.org/10.1152/physrev.00036.2017
Thebti, A., Meddeb, A., Ben Salem, I., Bakary, C., Ayari, S., Rezgui, F., … Ouzari, H.-I. (2023). Antimicrobial Activities and Mode of Flavonoid Actions. Antibiotics, 12(2), 225. https://doi.org/10.3390/antibiotics12020225
Tsanova-Savova, S., Denev, P., & Ribarova, F. (2018). Flavonoids in Foods and Their Role in Healthy Nutrition. In Diet, Microbiome and Health (pp. 165–198). Elsevier. Retrieved from https://linkinghub.elsevier.com/retrieve/pii/B9780128114407000077
Waditzer, M., & Bucar, F. (2021). Flavonoids as Inhibitors of Bacterial Efflux Pumps. Molecules, 26(22), 6904. https://doi.org/10.3390/molecules26226904
Waheed Janabi, A. H., Kamboh, A. A., Saeed, M., BiBi, J., Naveed, M., & Huixia, L. (2019). Flavonoid-rich foods (FRF): A promising nutraceutical approach against lifespan-shortening diseases. Iranian Journal of Basic Medical Sciences, (Online First). https://doi.org/10.22038/ijbms.2019.35125.8353
Wang, Y.-H., Dong, H.-H., Zhao, F., Wang, J., Yan, F., Jiang, Y.-Y., & Jin, Y.-S. (2016). The synthesis and synergistic antifungal effects of chalcones against drug resistant Candida albicans. Bioorganic & Medicinal Chemistry Letters, 26(13), 3098–3102. https://doi.org/10.1016/j.bmcl.2016.05.013
Weston, L. A., & Mathesius, U. (2013). Flavonoids: Their Structure, Biosynthesis and Role in the Rhizosphere, Including Allelopathy. Journal of Chemical Ecology, 39(2), 283–297. https://doi.org/10.1007/s10886-013-0248-5
Williams, R. J., Spencer, J. P. E., & Rice-Evans, C. (2004). Flavonoids: Antioxidants or signalling molecules? Free Radical Biology and Medicine, 36(7), 838–849. https://doi.org/10.1016/j.freeradbiomed.2004.01.001
Williamson, G., Kay, C. D., & Crozier, A. (2018). The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Comprehensive Reviews in Food Science and Food Safety, 17(5), 1054–1112. https://doi.org/10.1111/1541-4337.12351
Xian, D., Guo, M., Xu, J., Yang, Y., Zhao, Y., & Zhong, J. (2021). Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Report, 26(1), 134–146. https://doi.org/10.1080/13510002.2021.1962094
Xie, Y., Yang, W., Tang, F., Chen, X., & Ren, L. (2014). Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Current Medicinal Chemistry, 22(1), 132–149. https://doi.org/10.2174/0929867321666140916113443
Yuan, G., Guan, Y., Yi, H., Lai, S., Sun, Y., & Cao, S. (2021). Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Scientific Reports, 11(1), 10471. https://doi.org/10.1038/s41598-021-90035-7
Yuan, G., Xia, X., Guan, Y., Yi, H., Lai, S., Sun, Y., & Cao, S. (2022). Antimicrobial Quantitative Relationship and Mechanism of Plant Flavonoids to Gram-Positive Bacteria. Pharmaceuticals, 15(10), 1190. https://doi.org/10.3390/ph15101190
Zhang, L., Yan, Y., Zhu, J., Xia, X., Yuan, G., Li, S., … Luo, X. (2023). Quinone Pool, a Key Target of Plant Flavonoids Inhibiting Gram-Positive Bacteria. Molecules, 28(13), 4972. https://doi.org/10.3390/molecules28134972
Zhang, Q., Yang, W., Liu, J., Liu, H., Lv, Z., Zhang, C., … Jiao, Z. (2020). Identification of Six Flavonoids as Novel Cellular Antioxidants and Their Structure-Activity Relationship. Oxidative Medicine and Cellular Longevity, 1–12. https://doi.org/10.1155/2020/4150897
