Ciencia, Ambiente y Clima, Vol. 3, No. 1, enero-junio, 2020 • ISSN (impreso): 2636-2317 • ISSN (en línea): 2636-2333 • Sitio web: https://revistas.intec.edu.do/
EFFECT OF AN EXTRACT OF HIBISCUS SABDARIFFA L., ON OXIDATIVE STRESS INDUCED IN SACCHAROMYCES CEREVISIAE
Efecto de un extracto de Hibiscus sabdariffa L., sobre el estrés oxidativo inducido en Saccharomyces cerevisiae
Cómo citar: Pacheco Coello, F., Orosco-Vargas, C., Peraza-Marrero, M., Pinto-Catari, I., & Ramirez-Azuaje, D. (2020). Effect of an extract of Hibiscus sabdariffa L., on oxidative stress induced in Saccharomyces cerevisiae. Ciencia, Ambiente y Clima, 3(1), 41-46. Doi: https://doi.org/10.22206/cac.2020.v3i1.pp41-46
Introduction
Hibiscus sabdariffa L. (commonly known as roselle), is an annual or perennial plant with red stems and calyces, belonging to Malvaceae. It is cultivated mainly in the tropical and subtropical areas of both hemispheres (Patel, 2014). In Africa, roselle has two main uses: as a vegetable and for preparation of a beverage (Ghazala & Rajni, 2018). In Guinea-Bissau it is known as ondjo and both leaves and calyces are widely consumed and marketed (Ramírez-Rodrigues et al., 2012). The use of this plant comes from ancestral times and is mainly used in the preparation of hot and cold beverages mainly due to its proven antioxidant function in humans, especially against chronic diseases (Kao et al., 2016; Moyano et al., 2016). These effects have been associated to the presence of some phenolic compounds namely, anthocyanins (cyanidin derivatives), flavonols (quercetin and kaempferol derivatives), phenolic acids (chlorogenic acid) and also due to the presence of a specific organic acid from H. sabdariffa (hibiscus acid) (Borras-Linares et al., 2015).
A correlation between the total phenolic content of H. sabdariffa extracts and their antioxidant properties has been reported (Yang et al., 2012; Herranz-López et al., 2017). Therefore, it must be presumed that the antioxidant properties of the extracts lie on the polyphenol´s, abilities to scavenge free radicals. This effect has been observed in several oxidative damage models, in both cells and experimental animals. Many scientific investigations have revealed that the calyces of roselle are rich in polyphenols and flavonoids that enhanced the nutritive value of roselle as these compounds are correlated with their antioxidant property (Jabeur et al., 2017; Ismail et al.; 2018). All organisms have antioxidant defenses, including antioxidant enzymes, superoxide dismutase (SOD), catalase (CAT) and dietary components (Vitamin E, Vitamin C and beta-carotene) that are used to remove or repair damaged molecules (Halliwell, 1991; Ak & Gulcin, 2008; Stevanovic, et al., 2019).
Therefore, the study had the objective to evaluate the antioxidant potential of an abstract of hibiscus H. sabdariffa on S. cerevisiae in the presence of hydrogen peroxide.
Materials and methods
Origin of vegetal material
The vegetal material was donated by independent farmers in the City of Maracay, Aragua State, Venezuela (Geographical coordinates: 10° 14′ 49″ N, 67° 35′ 45″ W).
Preparation of aqueous extract
The extract was prepared with 2.5 g dry calyxes and 100 mL distilled water. The mixture was boiled for 15 min and the liquid separated from the calyxes by decantation. The extraction was repeated in the same conditions. The extracts were filtered together through Whatman No. 4 filter paper and gauged to 200 mL with distilled water (Reyes-Luengas et al., 2015).
Determination of total phenolics
The determination of phenols was carried out by the method colorimetric Folin-Ciocalteu. A volume of 50 μL of extract was mixed with125 μL of the Folin reagent, and 400 μL of sodium carbonate 7.1% (w/v), supplemented with distilled water to 1 mL. This procedure was performed in quintuplicate. Then, 5 concentration standards of 50, 100, 150, 200 and 250 μg/mL were prepared from a standard stock solution of Gallic Acid (phenol) with a concentration of 500 μg/mL. Finally, the reading was performed at 760 nm using the molecular absorption equipment Genesis 20 (Thermo Scintific). The results were expressed as mg of GAE / g of vegetal material (VM) (Singleton & Rossi, 1965).
Oxidative stress test on Saccharomyces cerevisiae
This assay seeks to evaluate the ability of the compounds present in the extracts to promote the growth of yeast when subjected to oxidative stress with hydrogen peroxide, for which the following experimental sequence was carried out (Alvarez et al., 2012): The positive control was performed with ascorbic acid, the negative without antioxidant and a blank only with yeast and culture medium. Before starting the absorbance measurements, S. cerevisiae was inoculated for 24 h under agitation at 37 ° C with 8 mL of YPD culture medium (peptone 2% w / v, glucose 2% w / v), 25 μL of yeast stock (solution of S. cerevisiae with a cell concentration of 4.5 x 106 cells / mL), 100 μL of ascorbic acid (control positive) and 80 μL of the extract of the samples. In control negative and white were inoculated only 8 mL of YPD and 25 μL of the yeast stock. After this incubation time of the yeast under the different conditions the absorbance was measured at 600 nm, this was approximately 0.240 and corresponded to the starting point of latency period of S. cerevisiae. Then 160 were added μL of 1 mM hydrogen peroxide to the positive control, negative and the extract to be evaluated at different concentrations. It was incubated at 37 ° C under stirring for 6 h and readings were taken every 30 min. Before each reading they waved the glass tubes for 10 s in vortex to ensure the homogeneity of the cells in the culture medium, then 100 μL of each test was taken and then taken to plates 96 wells Orbital shaking was performed before each reading 20 s and the absorbance readings were taken at 600 nm.
Statistical analysis
Analyses were done in triplicate, and the results were expressed as means ± standard deviation (SD). Results of antioxidant activity were each subjected to analysis of variance (ANOVA).
Results and Discussion
The concentration of total phenols the aqueous extract of H. sabdariffa was of 119 µg/mL (11.8 mg of GAE / g VM). The results show that the pure and diluted extract was able to inhibit the oxidative stress originated, with significant statistical difference in relation to the positive control (figure 1). The observed effect is due to the fact that the phenolic compounds present in the extract can indirectly regulate the expression and activity of enzymes such as CAT, SOD and glutathione (Ray et al.,2012) (Figure 2).
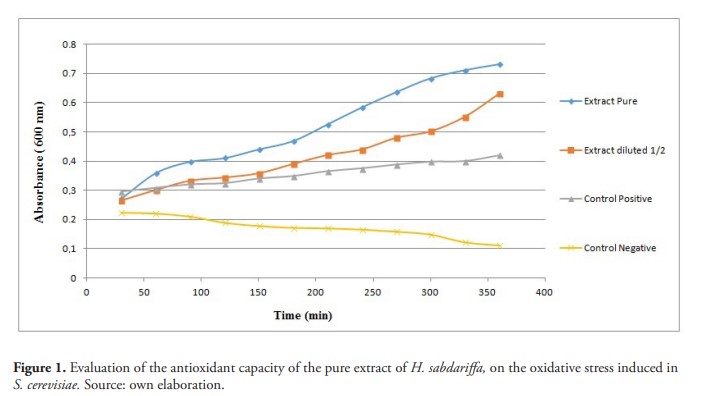
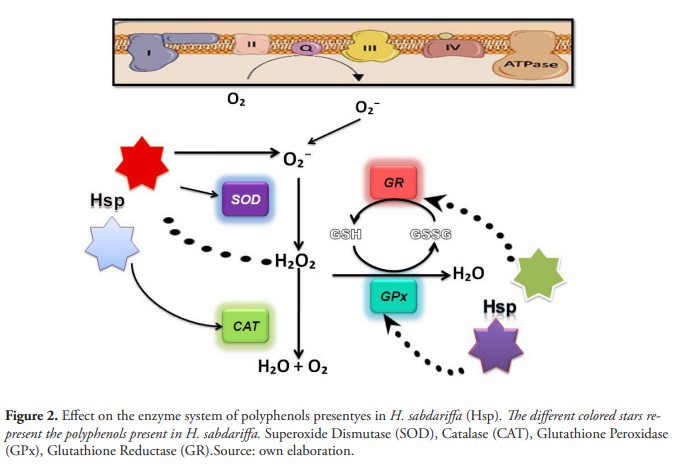
The results coincide with what was found in ethanolic extracts of Hibiscus tiliaceus L, which conferred protection to various strains of S. cerevisiae strains defective in antioxidant defenses against H2O2 and t-BOOH cytotoxicities, showing a clear antioxidant activity. These results suggesting that protection may be due to molecules that act as versatile and wide spectrum no enzymatic antioxidants, such as vitamins or phytosterols (Rosa et al., 2006).
Natural antioxidants are an interesting alternative in view of their variety of structures and chemical interactions, as well as the numerous biological activities they can perform. Intensive research activities are currently being carried out on plant antioxidants to meet this challenge (Kasangana et al., 2015).
Conclusions
H. sabdariffa showed a high antioxidant potential even in low concentration. This study is an important issue in the sense of what this polyphenol-rich silver represents, using S. cerevisiae as a biological model.
Acknowledgements
The authors would like to thank the Center for the Study of Workers’ Health at the University of Carabobo and the Saber Cell Biotechnology Laboratory.
References
Ak, T., & Gulcin., I. (2008). Antioxidant and radical scavenging properties of curcumin. Chemistry Biology Interaction, 174, 27-37.
Alvarez, E., De la Rosa, L., Amarowicz, R., &Shahidi, F. (2012). Protective effect of fresh and processed Jalapeño and Serrano peppers against food lipid and human LDL cholesterol oxidation. Food Chemistry, 133, 827-834.
Borras-Linares, I., Fernández-Arroyo, S., Arraez-Roman, D., Palmeros-Suárez, P.A., Val-Diaz, D.R., Andrade-Gonzales, I., Fernández-Gutierrez, A., Gómez-Leyva, J.F., & Segura-Carretero, A. (2015). Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican roselle (Hibiscus sabdariffa). Industrial Crops and Products, 69, 385-394.
Ghazala, R., & Rajni, C. (2018). A review on phyto-chemistry and therapeutic uses of Hibiscus sabdariffa L.Biomedicine Pharmacotherapy, 102(1), 575-586.
Halliwell, B. (1991). Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. American. Journal Medicine, 91, S14-S22.
Herranz-López, M., Olivares-Vicente, M., Encinar, J., Barrajón-Catalá A., Segura-Carretero, A., Joven, J., Micol, V. (2017). Multi-targeted molecular effects of Hibiscus sabdariffa polyphenols: an opportunity for a global approach to obesity. Nutrients,9, (8), 907-916.
Ismail, E.H.K., Ikram, H.S.M., & Nazril, T.G.S. (2018). Roselle (Hibiscus sabdariffa L.) seeds- nutritional composition, protein quality and health benefits. Food, 2(1), 1-16.
Jabeur, I., Pereira, E., Barros, L., Calhelha, R.C., Soković, M., Oliveira, M.B.P., & Ferreira, I.C.F.R. (2017). Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Research International, 100, 717-723.
Kao, E-S., Yang, M-Y., Hung, C-H., Huang, C-N.,Wang, C-J. (2016). Polyphenolic extract from Hibiscus sabdariffa reduces body fat by inhi-biting hepatic lipogenesis and preadipocyte adipogenesis. Food & Function, 7(1), 171-182
Kasangana, P., Haddad, P., & Stevanovic, T. (2015). Study of Polyphenol Content and Antioxidant Capacity of Myrianthus Arboreus (Cecro-piaceae) Root Bark Extracts, Antioxidants, 4, 410-426.
Moyano, G., Sáyago-Ayerd, S.G., Largo, C., Caz, V., Santamaria, M., & Tabernero, M. (2016). Potential use of dietary fibre from Hibiscus sabdariffa and Agave tequilana in obesity mana-gement. Journal of Functional Foods, 21, 1-9.
Patel, S. (2014). Hibiscus sabdariffa, an ideal yet under-exploited candidate for nutraceutical applications. Biomedicine and Preventive Nutri-tion, 4(1), 123-137.
Ramírez-Rodrigues, M.M., Plaza, M.L., Azeredo, A., Balaban, M.O., & Marshall, M.R. (2012). Phytochemical, sensory attributes and aroma stability of dense phase carbon dioxide processed Hibiscus sabdariffa beverage during storage. Food Chemistry, 134, 1425-1431.
Reyes-Luengas, A., Salinas-Moreno, Y., Ovan-do-Cruz, M., & Arteaga-Garibay, R. (2015). Analysis of phenolic acids and antioxidant activity of aqueous extracts of jamaica (Hibiscus sabdariffa L.) varieties with calyxes of different colors , Agrociencia, 49, 277-290.
Ray, P.D., Huang, B.W., & Tsuji, Y. (2012). Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cells Signal, 24, 981–990.
Rosa, R.M., Melecchi, M.I., & Halmenschlager, F.C. (2006). Abad, Antioxidant and anti-mutagenic properties of Hibiscus tiliaceusmethanolic extract. Journal. Agriculture Food Chemistry, 54, 7324-7330.
Singleton, VL., & Rossi, JA. (1965). Colorimetry of total phenolics with phosphomolybdic–phos-photungstic acid reagents. American Journal ofEnology and Viticulture, 16(3), 144-158.
Stevanovic, T., Diouf, N., & Garcia-Perez, M. (2019). Bioactive polyphenols from healthy diets and forest biomass, Current Nutrition Food Science, 5:264-295.
Yang, L., Gou, Y., Zhao, T., Zhao, J., Li, F., Zhang, B., & Wu, X. (2012). Antioxidant capacity of extracts from calyx fruits of roselle (Hibiscus sabdariffa). African JournalBiotechnology, 11, 4063-4068.

